3517
eIRIS — An Eigen-Analysis Motivated Approach for More Robust Multi-Shot Diffusion-Weighted Imaging1Guangdong Provincial Key Laborary of Medical Image Processing, School of Biomedical Engineering, Southern Medical University, Guangzhou, People's Republic of China, 2Philips Healthcare, Guangzhou, People's Republic of China, 3Philips Healthcare (Suzhou), Suzhou, People's Republic of China, 4Neusoft Medical System, Shanghai, People's Republic of China
Synopsis
IRIS corrects the motion-induced inter-shot phase errors for multi-shot diffusion-weighted imaging by extracting the phase information from an additional navigator data, which may cause the distortion mismatch between the image and navigator data. To solve the distortion mismatch issue in IRIS without using B0 field map, we propose to extract the coil sensitivities and phase information from navigator data by using an eigen-analysis scheme. The performance of the proposed method is demonstrated in both phantom and in vivo data sets.
Purpose
Multi-shot EPI has been widely used for high spatial resolution diffusion-weighted MR imaging. However, motion-induced phase errors among shots might result in ghost artifacts which degrade the image quality for diagnosis. MUSE [1] and IRIS [2] have been proposed to correct these motion-induced phase variations in image space. Since MUSE uses SENSE reconstruction [3] of each shot to extract the phase information, the applicability of MUSE is limited by the number of shots due to SENSE g-factor. Compared with MUSE, IRIS has no such limitation since the phase information is from an additional navigator. Therefore, IRIS is preferred, especially when a large number of shots are required. However, IRIS has a potential problem due to the usage of navigator: the distortion of the image and the navigator data might be mismatched as discussed in IRIS paper. To solve this issue, original IRIS uses a B0 field map to correct the navigator distortion which takes extra scan time. Recently, a k-space reconstruction method, SEPARATE [4], was published to avoid the necessary of B0 field map. However, the k-space method intrinsically has lower signal-noise-ratio (SNR) than image domain scheme. The goal of this study is to develop an image domain reconstruction approach to solve the distortion mismatch issue in IRIS without using B0 field map, nor decreasing the SNR for multi-shot acquisition.Theory and Methods
An eigen-analysis based scheme for coil sensitivity maps (CSM) extraction, which is noticed to be more robust than conventional image space scheme, was recently validated in ESPIRIT [5]. Inspired by the scheme, two approaches are proposed in this work to improve IRIS. First, a virtual coil concept is used. Data from different shots are treated as virtual coil elements with the coil sensitivities modulated by motion-induced phase errors. Hence, the final image can be generated using typical SENSE reconstruction. Second, the eigen-analysis scheme is used to extract the modulated CSM that contain coil sensitivities and inter-shot phase variations from IRIS navigator data. The proposed method is named as eigen-analysis IRIS (eIRIS).
To validate the proposed method, both phantom and in vivo data sets were acquired on a Philips Multiva 1.5T scanner (Philips Healthcare, Suzhou, China), using an eight-channel head coil and a dual refocusing spin-echo multi-shot EPI pulse sequence [2]. The second 180◦ refocusing pulse was used to acquire IRIS navigator. For the phantom acquisition, the scan parameters include: number of shot (NS) =4, SENSE acceleration factor (R) =1, field of view (FOV) =230×230mm2, voxel size=1.20×1.20×5mm3, b=1000s/mm2, number of signal averages (NSA) =1, repetition time/echo time (TR/TE) =2036.4ms/87.4ms and echo-train=34 for image-echo, TE=135ms and echo-train=28 for navigator-echo. For the in vivo acquisition, the scan parameters include: NS=4, R=1, FOV=240×240mm2, voxel size=1.88×1.88×5mm3, b=500s/mm2, NSA=1, echo-train=30, TR/TE=1500.0ms/81.3ms for image-echo, TE=120.0ms for navigator-echo. For comparison, we implemented conventional IRIS without B0 correction (cIRIS) and SEPARATE.
Results
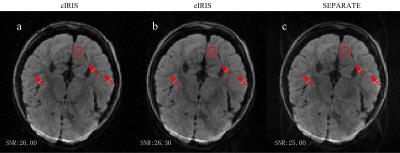
Figs.1 and 2 provide the results of phantom and in vivo data, respectively. The numbers in the figures are the quantitative measures of SNR within a region of interest (the red box in each image). They were calculated by dividing the mean of the signal by its standard deviation. Due to the distortion mismatch, cIRIS results in images with clear artifacts, as shown by the white arrows at images. SEPARATE avoids the artifacts in cIRIS, but reduces the SNR and introduces some residual aliasing artifacts. eIRIS results in the lowest noise/artifact level.Discussion
In this work, an eigen-analysis scheme is used to extract the modulated CSM to correct phase inconsistency in IRIS. Compared to cIRIS, eIRIS avoids the requirement of B0 field map, while results in the artifact-free images when there are distortion mismatch between navigator and image data. It hints that the eigen-analysis scheme for CSM extraction may be insensitive to the distortion. Compared to SEPARATE, it results in lower noise/artifact level due to the inheritance of the advantages of SENSE by the adoption of virtual coil elements concept.Conclusion
eIRIS solves the distortion mismatch issue in IRIS without using B0 field map, nor decreasing the SNR for multi-shot acquisition. Moreover, the CSM in eIRIS is from the navigator, thus it has the potential to avoid the motion-induced mismatch between reference scan and imaging data.References
[1] Chen N-k, et. al. NeuroImage 2013; 72:41-47. [2] Jeong H-k., et. al. MRM 2013; 69:793-802. [3] Pruessmann K.P., et. al. MRM 1999; 42:952-962. [4] Ma, Xiaodong, et. al. ISMRM 2015; p2799. [5] Uecker, M, et. al. MRM 2014; 71:990-1001.Acknowledgements
No acknowledgement found.References
No reference found.Figures