3507
High b-value Diffusion-Weighted MRI for Detection of Interscapular Brown Adipose Tissue in Rodent Model1Research Resource Center, University of Illinois at Chicago, Chicago, IL, United States, 2Radiology, Northwestern University, Chicago, IL, United States, 3Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 4Radiology, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
Brown adipose tissue (BAT) is increasingly considered a target organ for the treatment of metabolic disease. Persuasive evidence has shown that enhancement of the function of brown and beige adipocytes in humans could be very effective for treating type 2 diabetes and obesity. However, clinical studies have been limited by the lack of non-invasive tools for characterizing this tissue in humans. In this study, we explored the feasibility of using high b-value diffusion-weighted MRI for detecting the distribution of interscapular brown adipose tissue.
PURPOSE
Brown adipose tissue (BAT) is now considered a potential target for the treatment of obesity and obesity-related diseases in humans, including type 2 diabetes, atherosclerosis and lipid disorders.1 Diffusion-weighted magnetic resonance spectroscopy has been used to probe lipid droplet microstructures by characterizing changes in the diffusion of lipid protons.2, 3 However, MRI detection of BAT is needed to quantify biodistribution and to evaluate the impact of BAT targeted therapies. The objective of this study was to investigate the potential to visualize interscapular BAT (iBAT) with high b-value diffusion-weighted MRI at 9.4 T in a rodent model.METHODS
All studies were performed using a 9.4T 31 cm bore Agilent MRI scanner (Santa Clara, CA) with a 38 mm quadrature transceiver mouse coil. A test phantom was made using corn oil (control) and four samples of BAT dissected from the interscapular region (iBAT) of male mice (C57BL/6, approved by the IACUC). A diffusion-weighted stimulated echo sequence was used with following acquisition parameters: TR/TE = 2000/30.5 ms, mixing time = 382 ms, δ = 11 ms, Δ = 400 ms, slice thickness = 1.5 mm, field of view (FOV) = 36 mm × 36 mm, matrix = 64 × 64, average = 25, and 53 b-values ranging from 0 to 858,022 s/mm2 with a maximal diffusion gradient strength of 50 Gauss/cm. An ex vivo mouse was examined using the same sequence with the same parameters except 30 b-values ranging from 0 to 858,022 s/mm2, FOV of 36 mm × 50 mm, and matrix of 64 × 96. Anatomic T2-weighted images were acquired using a fast spin echo sequence with the following parameters: TR/TE = 1000/10 ms, echo train length = 8, matrix = 256 × 256, FOV = 36 × 50 mm, slice thickness = 1.5 mm, averages = 2. Image post-processing was performed in Matlab (MathWorks). Signal-noise-ratios (SNRs) were calculated from region of interest (ROIs) manually selected within each phantom sample and from ROIs drawn on iBAT and subcutaneous white adipose tissue (sWAT). Color-coded high b-value diffusion weighted images were co-registered and overlaid on the T2 weighted images to highlight the voxel-wise changes at high b-values.RESULTS
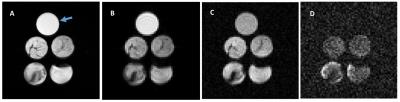
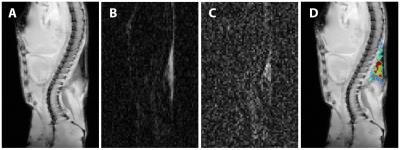
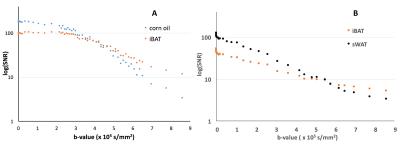
Relatively lower and more heterogeneous signals were observed for each of the 4 sample phantoms (Fig.1A), when compared to the corn oil in T2 weighted images. However, on the diffusion-weighted images (Fig. 1B, C, D), when compared to the corn oil, the four iBAT samples showed relatively lower signal intensities at low b values (b = 2.15 × 103 s/mm2, Fig.1B), equivalent signals at b = 4.20 × 105 s/mm2 (Fig. 1C), and clearly higher signals at b value of 8.58 × 105 s/mm2 (Fig. 1D), (b-value at which corn oil signal was no longer visible). As expected, only adipose tissues were visible in the heavy diffusion weighted images (Fig. 2B, C) of the ex vivo mouse. An anatomic image with color-coded overlay of the diffusion weighted image (b = 8.58 × 105 s/mm2) shows that the only remaining signal was from areas corresponding to iBAT (Fig. 2D). We further found that the iBAT signal decayed slower than the corn oil (Fig. 3A) and slower than the sWAT (Fig. 3B) as diffusion-weighting increased.DISCUSSION
The clinical ability to quantify BAT deposition would provide enormous benefits permitting in vivo assessment of whether a brown adipocyte phenotype has been achieved by pharmacological intervention. We found that the low diffusivity of iBAT could be used for iBAT detection with high b-value diffusion MRI, in which only signals from tissues with slow diffusion remained visible. In addition, iBAT in both phantom and mouse showed a non-linear relationship between the log-scaled SNRs and b-values (Fig. 3) indicating non-mono-exponential diffusion MR signal behavior and the existence of possible multiple diffusion compartments, which could offer the potential to use diffusion-model-based approaches for quantification of adipose tissues in future studies.CONCLUSION
In the current study, we demonstrated the feasibility of using high b-value diffusion-weighted MRI for detection of iBAT. Additional preclinical studies are on-going to optimize scanning parameters for improved sensitivity, and provide both additional histological validation and comparisons to other methods, e.g., 18F-FDG-PET,4 fat-fraction imaging methods,5, 6 and intermolecular zero-quantum MRI.7 Further studies are needed to validate this method for translational purposes since the high b-values used in this study may be difficult to achieve in clinical settings.Acknowledgements
No acknowledgement found.References
1. Harms, M. and P. Seale, Brown and beige fat: development, function and therapeutic potential. Nat Med, 2013. 19(10): p. 1252-63.
2. Xiao, L. and E.X. Wu, Diffusion-weighted magnetic resonance spectroscopy: a novel approach to investigate intramyocellular lipids. Magn Reson Med, 2011. 66(4): p. 937-44.
3. Cao, P. and E.X. Wu, In vivo diffusion MRS investigation of non-water molecules in biological tissues. NMR Biomed, 2016.
4. Saito, M., et al., High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes, 2009. 58(7): p. 1526-31.
5. Hu HH, Perkins TG, Chia JM, Gilsanz V. Characterization of human brown adipose tissue by chemical-shift water-fat MRI. AJR Am J Roentgenol, 2013. 200(1): p. 177-83.
6. Smith DL, Jr., Yang Y, Hu HH, Zhai G, Nagy TR. Measurement of interscapular brown adipose tissue of mice in differentially housed temperatures by chemical-shift-encoded water-fat MRI. J Magn Reson Imaging, 2013. 38(6): p. 1425-33.
7. Branca RT, Zhang L, Warren WS, Auerbach E, Khanna A, Degan S, Ugurbil K, Maronpot R. In vivo noninvasive detection of Brown Adipose Tissue through intermolecular zero-quantum MRI. PLoS One, 2013. 8(9): p. e74206.
Figures