3502
Improved Detection of Spinal Cord Injury in Rats with Reduced Field of View (rFOV) and Orthogonal Filter-Probe (OFP) Diffusion Weighted Imaging (DWI)1Biophysics Graduate Program, Medical College of Wisconsin, Milwaukee, WI, United States, 2Medical Scientist Training Program, Medical College of Wisconsin, Milwaukee, WI, United States, 3Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 4Clement J Zablocki Veterans Affairs Medical Center, Milwaukee, WI, United States, 5Biomedical Engineering, Marquette University, Milwaukee, WI, United States
Synopsis
To improve image quality for investigation of spinal cord injury, a reduced field of view excitation scheme was implemented on a Bruker 9.4T imaging system. This was combined with an optimized orthogonal filter-probe diffusion weighted imaging sequence to improve specificity for axonal injury with a short acquisition time. Application in a rat spinal cord injury model showed sensitive detection of diffusivity changes 48 hours post-injury with improved image quality compared to a standard full field of view acquisition. These advances provide support for this technique as a potential biomarker of injury severity with the ability for translation into clinical systems.
Purpose
Sensitive detection of spinal cord injury (SCI) severity is an unmet clinical need. Diffusion weighted imaging(DWI) has shown promise as a noninvasive prognostic biomarker of axonal damage, which is the best predictor of functional outcome from SCI1. However, the presence of confounding edema, long acquisition times, and complicated analysis has limited clinical adoption of DWI for SCI. To improve image quality and specificity for injury, a reduced field of view (rFOV) sequence was coupled with orthogonal filter-probe (OFP) diffusion encoding in a rat model of SCI.Methods
A Bruker 9.4T Biospec was used for imaging rats in vivo with
a quadrature volume coil for transmission and 4-channel surface coil array for
reception. A reduced field of view
(rFOV) excitation scheme2 was implemented in ParaVision v6.1 using
an echo planar trajectory with 16 excitation Gaussian sub-pulses each with 0.5
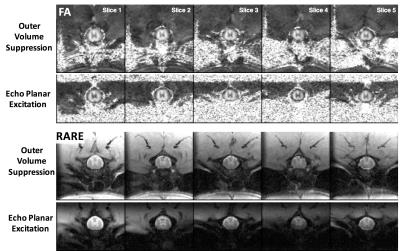
ms duration. The rFOV excitation, implemented for DWI-EPI and RARE sequences(13.5x9.6 mm2, 90x64 matrix), was compared to images of the same resolution (13.5x13.5 mm2, 90x90 matrix) obtained using outer volume suppression (OVS).
OVS included four 10 mm saturation bands surrounding
the acquisition field of view and a spectral-selective fat suppression pulse. rFOV did not
include any spatial or fat suppression.
DWI consisted of 12 directions, b-value of 800 s/mm2, with
TEs of 24 and 29 ms for the OVS and rFOV, respectively, and identical
acquisition times of 3:28 (with variation from respiratory gating). RARE images
used identical TE (20 ms; RARE factor=2) with acquisition times of 2:15 and
1:36 for the OVS and rFOV sequences, respectively. Both schemes had 150 μm2
in-plane resolution and 2 mm slice thickness.
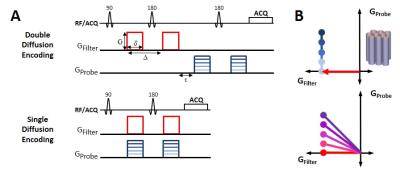
Separately, double diffusion encoding (DDE) was
compared to single diffusion encoding (SDE), sampling the identical points
in q-space(Fig. 1). The DDE-OFP
acquisition3 (TE= 61ms) consisted of two pairs of Stejskal-Tanner
gradients (δ/Δ=6/12 ms) with the first pair aligned orthogonal to the cord (b=2000 s/mm2) and the second pair aligned parallel to the cord with 5
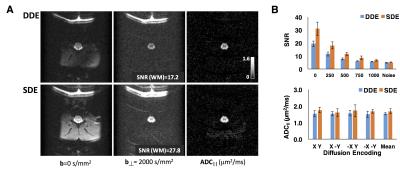
b-values ranging from 0-1000 s/mm2. The SDE (TE=35ms) consisted of identical OFP
diffusion encoding, but with gradient directions overlapped in time. A 4-shot EPI readout (TR=2s) with full-FOV
and OVS was used (25.6x25.6 mm2, 128x128 matrix). Maps of ADC|| along the spinal
cord were calculated from the “filtered” signals.
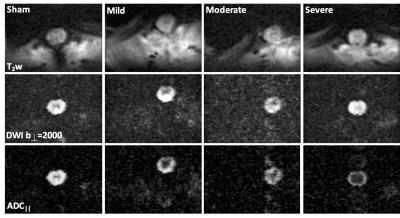
A combined rFOV-DWI sequence with the SDE-OFP scheme was then used in a rat model of contusion SCI at the T10 vertebral level at 48 hours post injury, with varying severity levels and an acquisition time of 5:36. ADC|| maps were evaluated using whole-cord region of interest (ROI) analysis.
Results
DWI and RARE images exhibited clear delineation of the spinal cord anatomy. rFOV image quality was comparable to OVS acquisitions, but improved over OVS imperfections causing signal aliasing, ghosting, and inadequate fat suppression (Fig. 2). Signal to noise ration(SNR) of the rFOV-DWI (47.4) decreased by approximately 12% compared to the OVS-DWI (41.5) averaged across all diffusion weighted images from a whole-cord ROI. SNR of the rFOV-RARE (39.0) was increased compared to the OVS-RARE (28.0).ADC|| maps from the OFP diffusion encoding scheme were devoid of non-spinal cord signals, as anticipated3. Due to a reduction in echo time, the SDE preparation scheme exhibited an SNR 1.6 times greater than the DDE. This resulted in a slight but non-significant increase in the estimated white matter ADC|| (Fig. 3b). Furthermore, no significant differences were evident resulting from positive or negative combinations of perpendicular and parallel diffusion gradient encoding directions (Fig. 3b).
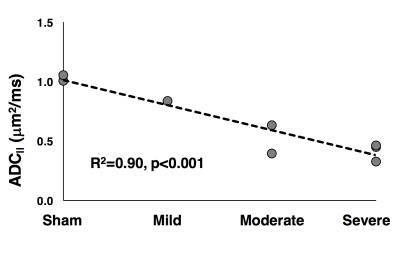
The combined rFOV and SDE-OFP scheme enabled high-resolution and high specificity for injury severity in the spinal cord(Fig. 4). Notably, in the severe SCI ADC|| map(Fig. 4c), a rim of peripheral white matter exhibits faster diffusivity compared to the slowly-diffusivity central region, which is consistent with the pathology of contusion injury4,5 but was not evident in the T2-weighted (Fig. 4a) or perpendicular DWI images (Fig. 4b). Whole-cord diffusivity in a small cohort (n=8) showed a consistent trend of decreasing ADC|| with increasing injury severity (R2=0.90; p<0.001).
Discussion
In the rat spinal cord, rFOV enables high-resolution images with no obvious penalty in image quality or acquisition time. As shown for human spinal cord DWI2, the intrinsic fat suppression and reduction of artifacts improves DTI protocols. Likewise, the OFP diffusion using SDE improves SNR while maintaining specificity for acute axonal injury.Conclusion
The OFP rFOV-DWI allows sensitive estimation and visualization of diffusion reflective of SCI severity without prominent artifacts or confounding pathologies. This improvement over traditional anatomic imaging techniques such as with a T2-weighting supports its potential as a noninvasive marker of SCI severity compatible with clinical translation.Acknowledgements
Project was partially funded through the Research and Education Initiative Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin, the Craig H. Neilsen Foundation, and the Department of Veterans Affairs. NS is a member of the Medical Scientist Training Program at MCW, which is partially supported by a training grant from NIGMS T32-GM080202. NS is partially supported by the National Center for Advancing Translational Science, National Institutes of Health, through grant numbers UL1TR001436 and 1TL1TR001437. Support from the Bryon Riesch Paralysis Foundation is gratefully acknowledged. Special thanks to Natasha Beucher and Kyle Stehlik for experimental assistance.References
1. Medana, I. M. and M. M. Esiri. Axonal damage: a
key predictor of outcome in human CNS diseases. Brain. 2003; 126(Pt 3):
515-530.
2. Saritas E, Cunningham C, Lee J, et al. DWI of
the Spinal Cord with Reduced FOV Single-Shot EPI. Magn Reson Med. 2008;
60:468-473.
3. Skinner N, Kurpad S, Schmit B, et al. Rapid In
Vivo Detection of Rat Spinal Cord Injury with Double-Diffusion-Encoded Magnetic
Resonance Spectroscopy. Magn Reson Med. In Press.
4. J Kim, D Loy, H Liang, et al. Noninvasive Diffusion Tensor Imaging of
Evolving White Matter Pathology in a Mouse Model of Acute Spinal Cord Injury.
Magn Reson Med. 2007; 58:253-260.
5. O Hausmann. Post-traumatic Inflammation
following Spinal Cord Injury. Spinal Cord. 2003; 41:369-378.
Figures