3483
Structural abnormalities in frontal lobe pathways in children with Attention-Deficit/Hyperactivity Disorder (ADHD)1College of Computer Science and Technology, Harbin Engineering University, Harbin, People's Republic of China, 2Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States, 3Department of Radiology, Children's Hospital of Boston, Boston, MA, United States, 4Department of Psychiatry, Children's Hospital of Boston, Boston, MA, United States, 5Fetal-Neonatal Neuroimaging and Developmental Science Center, Children's Hospital of Boston, Boston, MA, United States, 6Scientific Review and Behavioral Science Core, Children's Hospital of Boston, Boston, MA, United States
Synopsis
Structural abnormalities in frontal lobe connections have been observed in adults/children with ADHD in earlier studies using diffusion tensor imaging (DTI)3. This abstract investigates microstructural differences in frontal-lobe white matter connectivity using advanced diffusion imaging methods. 47 white matter fiber bundles connecting frontal areas as parcellated by Freesurfer were extracted using a novel whole-brain tractography algorithm4,1, which allowed estimation of specific diffusion properties such as cellular volume and cellular density from advanced diffusion MRI (dMRI) data. After correcting for multiple comparisons, 6 significant white matter pathways were found to have lower cellular volume and density in ADHD compared to controls.
Purpose
Attention-deficit/hyperactivity
disorder (ADHD) is a highly prevalent developmental disorder among school-age
children and adults characterized by inattention, hyperactivity and impulsivity.
Studies using standard DTI models have reported lower FA (fractional
anisotropy) in the frontal white matter areas along with the superior
longitudinal fasciculus II (SLF II), which are involved in attention, memory
and higher level cognitive thinking. However, DTI has several limitations,
including inability to model crossing fibers. Further, FA is a very
non-specific measure of white matter integrity. In this abstract, we use
multi-shell diffusion imaging to trace and analyze crossing fibers, along with
estimating specific dMRI measures of cellular volume and density in these
tracts to determine specific structural deficits in children with ADHD.Methods
MRI acquisition: Our study included: 30 subjects with ADHD (Female/Male: 7/23, mean age: 10.6447 yrs) and 28 age-matched controls (Female/Male: 10/18, mean age: 10.6054 yrs). Diffusion MRI data was acquired using multi-slice acquisition (x2) at spatial resolution of 2x2x2mm3; with 70 gradient directions spread over 3 b-value shells of 1000/2000/3000s/mm2. Semi-automated quality control was performed on all data sets and all gradients with signal drop were removed. Eddy current and head motion correction was performed using in-house scripts. Extraction of frontal lobe connections: We used our multi-fiber UKF-tractorgraphy4 algorithm with a bi-exponential model to simultaneously estimate the model parameters and perform tractography. The method allowed computation of parameters such as the return-to-orientation probability (RTOP) and return-to-axis probability (RTAP), which are inversely proportional to cellular volume and cellular cross-sectional area (or axonal density). These specific parameters were never investigated in ADHD before, which is one of the major contributions of this work. 47 white matter frontal connections were extracted from whole-brain tractography using the white matter query language (WMQL)4 with brain parcellations obtained using FreeSurfer software2. Statistical analysis: Linear discriminant analysis (LDA) was first used to maximally separate the two groups with average measures of RTOP and RTAP obtained for each fiber bundle. These were then projected onto the normal of the decision boundary to obtain a univariate measurement, on which a two-sample t-test was performed followed by FDR correction for multiple comparison. Six statistically significant white-matter connections survived the multiple comparisons.Results
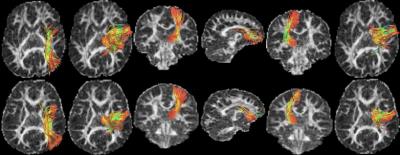
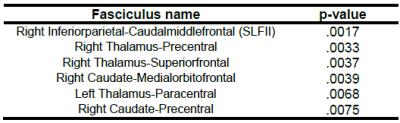
The 6 fiber bundles found to be abnormal in ADHD, i.e., having lower cellular volume and density are: 1) right SLF-II, 2) right thalamus to right precentral, 3) right thalamus to right superiorfrontal, 4) right caudate to right medial-orbitofrontal, 5) left thalamus to left paracentral and 6) right caudate to right precentral. The p-values are given in Table 1, and the obtained fiber tracts shown in Figure 1.Discussion
Our results show that the thalamo-frontal connections, the caudal-frontal and the right SLF-II white matter fibers are affected in children with ADHD. Specifically, to the best of our knowledge, this is a first study that has reported differences in more specific measures of cellular volume and density in complex white matter structures in ADHD. Past results have reported differences in FA and radial diffusivity in SLF-II and other white matter regions. However, these measures are non-specific and do provide a clearer indication of the abnormalities in these regions. The thalamus plays a critical role in self-regulation and has been a target for ADHD treatment5. Moreover, it also supports the language systems, and has an extended memory function, which are also affected in ADHD. Our finding confirms earlier results on abnormalities in the right SLF-II of individuals with ADHD, which is involved in spatial attention and executive function. Our results show that this pathway has lower cellular volume and density, leading to pathology in the attention network. Earlier results have shown a smaller caudate nucleus (right) in children with ADHD. We show that the white matter connections of the caudate to the frontal lobe might also be involved. The caudate plays an important role in learning, memory and inhibitory control, aspects which are also phenotypes affected in ADHD.Acknowledgements
This work is supported by Natural Science Foundation of China under Grant No.61502117, Science Foundation of Heilongjiang Province under Grant No.QC2016084 and National Institutes of Health under Grant R01MH099797 (PI: Rathi).References
1. J. G. Malcolm, M. E. Shenton, and Y. Rathi. Filtered multitensor tractography. IEEE Trans. Med. Imaging. 2010;29(9):1664-1675.
2. B. Fischl. FreeSurfer. Neuroimage. 2012;62(2):774-781.
3. L. Ning, C-F. Westin, and Y. Rathi. Estimating diffusion propagator and its moments using directional radial basis functions. IEEE Trans. Med. Imaging. 2015;34(10):2058-2078.
4. Y. Rathi, B. Gagoski, K. Setsompop, O. Michailovich, P. Ellen Grant, and C-F Westin. Diffusion propagator estimation from sparse measurements in a tractography framework. In International Conference on Medical Image Computing and Computer-Assisted Intervention, pp. 510-517. Springer Berlin Heidelberg, 2013.
5. T. Bailey, and A Joyce. The role of the thalamus in ADHD symptomatology and treatment. Applied Neuropsychology: Child. 2015;4(2):89-96.
Figures