3471
Reproducibility of SMT-Based Microscopic Diffusion Anisotropy Imaging on a Clinical MRI System1Developmental Imaging & Biophysics, University College London, London, United Kingdom, 2Centre of Medical Image Computing, University College London, London, United Kingdom
Synopsis
Diffusion Tensor Imaging (DTI) is a widely used neuroimaging technique, but it lacks specificity. Advanced diffusion models aim to yield greater biophysical information than DTI. This information is redundant if the parameters cannot be accurately reproduced across time-points. Multi-shell diffusion images were acquired on a single 3T scanner at two time-points for ten healthy adults. Mean parameter values from SMT-based microscopic diffusion anisotropy imaging and DTI were obtained in white matter, cortex and thalami. The advanced model performed similarly well to DTI in white matter, but was less consistent in the cortex and thalami, potentially due to its increased complexity.
Purpose
The purpose of this study was to examine the reproducibility of multi-shell diffusion measures on a single scanner between two time-points. Diffusion Tensor Imaging (DTI) parameters were analysed, along with parameters from the multi-compartment Spherical Mean Technique (SMT) diffusion model1,2.
DTI is widely used in neuroimaging but it lacks specificity; the derived measures do not allow the unambiguous inference of unique microstructural information. In contrast, the SMT model attempts to directly estimate tissue microstructure without knowledge of neurite orientation distribution and is unconfounded by fibre crossings.
Advanced diffusion models have greater biophysical specificity than DTI, but due to their increased complexity, they may be less reproducible. Examining the reproducibility of these measurements is important to assess their clinical and neuroscientific utility. Here we assess the relative reproducibility of diffusion parameters measured with DTI (Fractional Anisotropy – FA; Mean Diffusion – MD) and parameters from the SMT diffusion model1,2 (Microscopic Fractional Anisotropy – Micro FA; Intrinsic Diffusivity; Intra-Neurite Volume Fraction; Mean Diffusivity of the Extra-Neurite Compartment) in a group of healthy adults scanned repeatedly using multi-shell diffusion imaging.
Methods
Ten healthy adult volunteers were scanned on a single scanner (Siemens Prisma 3T). Images were acquired using a single-shot EPI sequence. Diffusion gradients were applied in 60 directions each at b = 1000 smm-2 and 2200 smm-2. An additional 13 b = 0 images, and a final b = 0 with the phase-encode direction reversed, were acquired.
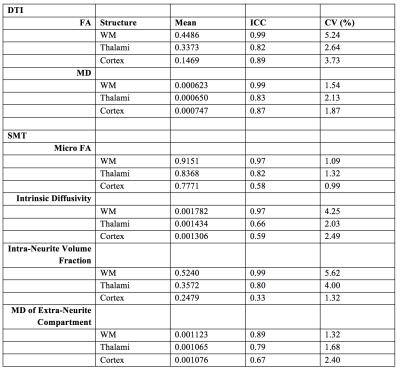
The mean DTI parameters of FA and MD were measured in the white matter (WM), cortex and thalami. The SMT parameters of microscopic FA, intrinsic diffusivity, intra-neurite volume fraction and the mean diffusivity of the extra-neurite compartment were measured in WM, cortex and thalami. A mixed-effect model was used to measure the intra-scanner reproducibility, stating values of Intra-Class Correlation (ICC). The Coefficient of Variation (CV) was calculated to establish variability.
Results
FA and MD had particularly high reproducibility in WM with both having respective ICC scores of 0.99. Overall, the reproducibility of the SMT parameters was good in WM but slightly lower than DTI, with ICCs ranging from 0.89 to 0.99.
The ICCs of all parameters were reduced in both the thalami and cortex. Despite this, mean values of FA and MD were largely reproducible in these regions with values ranging from 0.89 to 0.82. The reproducibility of the SMT metrics in the cortex and thalami was reasonable with ICC values ranging from 0.59 to 0.80.
The variability of each of the parameters across subjects in all of the regions was low, ranging from 0.99% to 5.62%.
Discussion
To our knowledge, this is the first study that assesses the reproducibility of the SMT model. DTI was shown to be the most reproducible method as expected due to its increased simplicity. However, SMT is similar in terms of reproducibility, whilst potentially improving specificity. Mean FA and MD showed consistently high ICC values in all regions, whilst the reproducibility of the SMT parameters was less consistent.
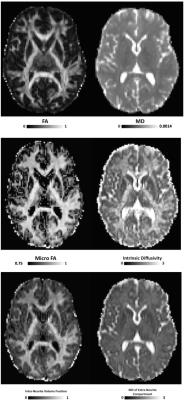
Mean values of all parameters are in agreement with the wider literature (Figure 1)1-6 and example images for a single subject for all measured parameters are shown in Figure 2. The high reproducibility of each of the techniques highlights their robustness, further indicating their potential suitability for translation into the clinic.
Overall, the results indicate that the SMT model is noisier than DTI, which is expected due its increased complexity. However, the reproducibility of SMT rivalled that of DTI in WM, which is particularly promising as it may potentially produce greater sensitivity to microstructural change.
Conclusion
The SMT diffusion model parameters have a scan-rescan reproducibility that is comparable to DTI in WM. SMT is less reproducible in the cortex and thalami compared to DTI. These results indicate that the SMT parameters are robust and reproducible. Future work will aim to explore the reproducibility of the SMT in other regions of the brain in comparison to DTI in order to further ascertain the potential for clinical utilisation.Acknowledgements
EPSRC CDT in Medical Image Computing at UCLReferences
1. Kaden E, Kruggel F, Alexander DC. Quantitative mapping of the per-axon diffusion coefficients in brain white matter. Magnetic resonance in medicine. 2016;75(4):1752-63.
2. Kaden E, Kelm ND, Carson RP, Does MD, Alexander DC. Multi-compartment microscopic diffusion imaging. NeuroImage. 2016;139:346-359.
3. Grech-Sollars M, Hales PW, Miyazaki K, et al. Multi-centre reproducibility of diffusion MRI parameters for clinical sequences in the brain. NMR Biomed. 2015;28:468–85.
4. Vollmar C, O’Muircheartaigh J, Barker GJ, Symms MR, Thompson P, Kumari V, Duncan JS, Richardson MP, Koepp MJ. Identical, but not the same: intra-site and inter-site reproducibility of fractional anisotropy measures on two 3.0T scanners. Neuroimage. 2010;51:1384–94.
5. Veenith T V, Carter E, Grossac J, Newcombe VFJ, Outtrim JG, Lupson V, Williams GB, Menon DK, Coles JP. Inter subject variability and reproducibility of diffusion tensor imaging within and between different imaging sessions. PLoS One. 2013;8:e65941.
6. Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WMA, Barker PB, Mori S, Horská A. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. NeuroImage. 2007;34:733–42.
Figures