3469
7.0 T Diffusion Tensor Imaging Evaluation of Rabbit Sciatic Nerve Microstructure with Histologic CorrelationTina Jeon1, Emil Stefan Vutescu2, Eric Aronowitz3, Henning U Voss3, Jonathan P Dyke3, and Darryl B Sneag1
1Radiology and Imaging, Hospital for Special Surgery, New York, NY, United States, 23Department of Hand and Upper Extremity Service, Hospital for Special Surgery, New York, NY, United States, 3Citigroup Biomedical Imaging Center, Weill Cornell Medical College, New York, NY
Synopsis
High-resolution DTI is a promising tool to evaluate peripheral nerve regeneration following surgical repair of nerve injury. The spatial resolution achieved with 7.0 T allowed us to more confidently interrogate the nerve for measuring fractional anisotropy (FA) and diffusivity and to perform fiber tracking as compared to 3.0 T. Among DTI metrics, FA correlated the greatest with axonal density and diameter. These findings support that DTI has the potential to measure axonal regeneration in the peripheral nerves at preclinical and clinical field strengths.
Purpose
Ex-vivo high resolution diffusion tensor imaging (DTI) of the rabbit sciatic nerve at 7.0T is able to capture complex fiber configurations such as sharp curves and convergence/divergence of tracts. Given the small diameter of the rabbit sciatic nerve (2-3 mm), sub-millimeter resolution is necessary to properly evaluate axonal integrity, without bias, to avoid partial volume averaging of complex fiber architecture and to improve accuracy of DTI-based fiber tracking.1 We investigated sciatic nerve regeneration following transection repaired with autograft with ex-vivo DTI correlated with histology.Methods
Surgical procedure: Under anesthesia, a 10 mm transection gap was created in the sciatic nerve of the proximal thigh of 24 adult male New Zealand white rabbits and the gap was bridged with either autograft or a synthetic conduit. In-vivo analysis was performed of all rabbits on 3.0T but for this ex-vivo study, 12 rabbit limbs were harvested and scanned (9 from non-operated (control) limbs and 3 autograft). Specimen Preparation: The excised sciatic nerve was fixed in phosphate buffer solution 24 hours before imaging. The nerve was then embedded in surgical gauze and placed in a MR-compatible plastic tube filled with Fomblin Grade 06/6. Data acquisition 7.0T: DTI was performed on a 7.0T Bruker BioSpin MRI with a 1 channel microimaging coil. 3D diffusion-weighted multi-shot spin echo EPI; TR/TE: 2000/30ms; spatial resolution: 0.15625 mm isotropic; b-values=0,2000 s/mm2; 1 acquisition; 60 gradient directions; imaging time=10hrs24 min; bandwidth=1171.875 MHz. Data acquisition 3.0T: DTI was performed on a 3.0T Siemens Healthcare Prisma with combined 8-ch flexible array coil placed anteriorly on a 32-channel spine coil placed anteriorly with the rabbit prone. DTI was acquired using a single-shot EPI with SENSE (SENSitivity Encoding reduction factor=2) parallel imaging scheme. FOV=130x75mm[DBS1] ; matrix=76x44mm, ST=1.7mm, TE/TR=47/3400ms, Acquisitions=10; 20 gradient directions, b-values=0,700 sec/mm2, total imaging time=11:42 mins. Sciatic nerve segmentation and white matter tracking: Tractography was performed with Trackvis (trackvis.org) using deterministic fiber tracking, turning angle < 30, FA threshold=0.10, and the tract then served as a mask for segmentation. DTI measurements: Tensor fitting was conducted with Diffusion Toolkit to obtain the DTI contrasts. Histopathological analysis: A 5 mm segment of the sciatic nerve was harvested from the operated and non-operated limbs distal to and at the surgical site. Samples were analyzed for axonal density and myelin fiber diameter. Correlation of in-vivo and ex-vivo DTI metrics with histology: Paired t-test was conducted between the DTI metrics for the in-vivo and ex-vivo study at 13 weeks post-op and between the non-operated and the operated nerve. Pearson correlation between the ex-vivo study and axon diameters and axonal density was performed with Bonferroni correction for multiple comparisons (alpha<0.05).Results
FA change on the operated limb: As shown in Fig.1, there was a significant FA decrease in the autograft 13 weeks postop compared to the operated limb (p<0.0004). Comparison between in-vivo and ex-vivo: There was a significant FA decrease with in-vivo studies compared to ex-vivo 7.0T (Fig.2a) and corresponding increases in the DTI contrasts, Fig.2b-d. Tractography: Fiber tracking can reveal fine details of the non-surgical and surgical limb (Fig.3), including a discontinuity in the nerve at the surgical site and consequently significantly reduced axonal diameters and axonal density distal to the surgical site. Correlation with histology: Figure 4 shows a tight correlation between FA and axonal diameter when the non-operated and operated nerves are combined (p<0.0024). Additionally, there is a significant correlation between FA and axonal density (p<0.016).Discussion and conclusion
This study’s results suggest that DTI is a sensitive measure of nerve microstructure in a rabbit sciatic nerve transection model and can provide DTI parameters that are in agreement with in-vivo studies and histology (Figs.1,2,4). FA is sensitive to cellular processes such as apoptosis, edema, axonal packing, demyelination and an increase in cellularity at the surgical site which changes grossly with nerve regeneration, resulting in lower FA values on the operated limb3,4. Lower FA and higher mean diffusivity values at 3.0T (Fig.2) compared to 7.0T could be due to partial volume effects near the borders of nerve/adjacent tissue. Furthermore, FA has been found to decrease with increasing signal-to-noise ratio (SNR), whereas MD decreases with increasing SNR5. Reliable fiber tracking with in-vivo 3.0T MRI was not possible due to inadequate spatial resolution; however, we were able to visualize them with ex-vivo 7.0T (Fig.3). Fiber tracking allows the opportunity to potentially elucidate the longitudinal extent of axonal regeneration thatcannot reliably be measured with electrophysiological testing. Given the recent interest in peripheral nerve imaging with DTI, we anticipate the further exploration of in-vivo and ex-vivo studies to assess microstructural changes to evaluate nerve regeneration.Acknowledgements
This study is sponsored by Radiology and Imaging Center at the Hospital for Special Surgery. We would like to thank Toyobo Co. Ltd. for funding the in-vivo study and the MRI technologist Eric Aronowitz.References
[1] Lehmann et al (2010) Exp Neurol 223: 238. [2] Boyer et al (2015) Neurosurgical Focus 39: E9. [3] Pierpaoli and Basser (1996) MRM 36:893. [4] Basser et al (1994) Biophys J 66: 259. [5] Landman et al (2008) MRI 29: 739.Figures
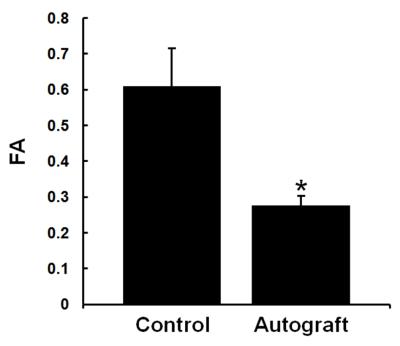
Figure 1:
Average FA values of sciatic nerves on the nonsurgical and
surgical side 13 weeks postop with Autograft. In comparison to the control
side, the FA is significantly reduced in the regenerated sciatic nerve segment
after surgery. Note: Nerbridge and Neuragen are not shown due to low SNR.
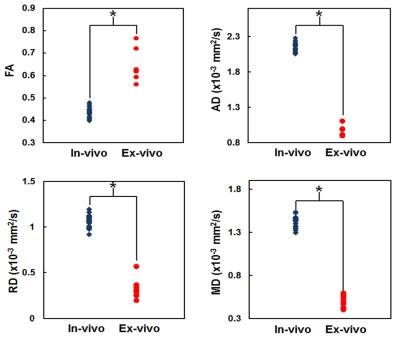
Figure
2: The FA (upper left), AD (upper right), RD (lower left), and
MD (lower right) of the in-vivo (blue) and ex-vivo (red) non-operated nerve.
Each dot represents one rabbit sciatic nerve. Asterisks indicate a statistical
significance after bonferroni correction.
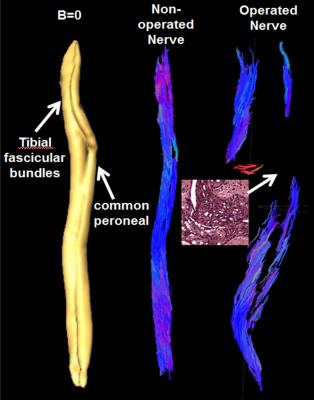
Figure 3:
Sagittal
view of the rabbit sciatic nerve reconstructed using the b=0 (left) and DTI
data (middle) from a non-operated nerve demonstrates clear definition of the
common peroneal and tibial fascicular bundles. Tractography from a nerve that
has been surgically operated on (right) shows a break in the tracking due to incomplete
nerve regeneration 13 weeks post-surgery. The histology from a nerve sample corresponding
to the conduit site in the inset confirms that there is sparse numbers of
regenerating axons.
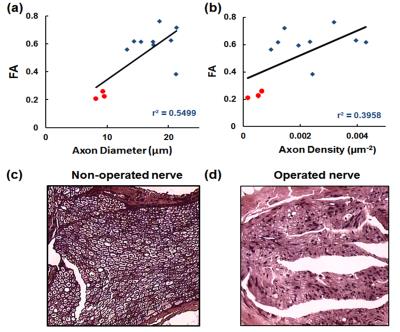
Figure 4: Quantitative
analysis of FA in non-surgical (blue) and autograft (red) with mean diameter of
axons (a) in the nerve and mean axonal density (b). There was a significant
correlation with FA and histological measures when the non-surgical and
surgical nerve is combined (r2=0.5499). Histologic sections of
distal portions of the sciatic nerve 13 weeks post-surgery of the non-operated
(c) and operated (d) nerve with hematoxylin-eosin staining at 100x
magnification. There is significantly less axons in the operated nerve.