3468
Hypoxia imaging of head and neck carcinoma: Correlation between DWI parameters and FAZA-PET activity1Applied MRI Research, Molecular Imaging and Theranostics, National Institute of Radiological Sciences, National Institutes for Quantum and Radiological Science and Technology, Chiba, Japan, 2Department of Oral and Maxillofacial Radiology, Tokyo Dental College, Tokyo, Japan, 3Department of Nuclear Medicine, Kawasaki Medical School, 4Department of Nuclear Medicine, Cancer Institute Hospital, Japanese Foundation for Cancer Research, 5Molecular Imaging and Theranostics, National Institute of Radiological Sciences, 6Department of Radiopharmaceuticals Development, National Institute of Radiological Sciences, 7National Institute of Radiological Sciences Hospital, 8Diagnostic Radiology and Radiation Oncology, Graduate School of Medicine, Chiba University, 9Department of Diagnostic Imaging and Nuclear Medicine, Kyoto University
Synopsis
To investigate the usefulness of diffusion-weighted imaging (DWI) for visualizing hypoxia of head and neck carcinoma, the correlation between DWI parameter estimates and 18F-fluoroazomycin arabinoside (FAZA) positron emission tomography (PET) activity was evaluated. The diffusion coefficients and fractions of the fast and slow compartments according to the 2-compartment model (Dfast, Dslow and Ffast, Fslow) were estimated. The diffusional kurtosis (K) and the corrected diffusion coefficient (D) were also obtained according to the diffusional kurtosis imaging (DKI) method. Amongst the DWI estimates, Dslow and K were significantly correlated with FAZA-PET activity, which suggests they might be useful as indicators of hypoxia.
Purpose
Hypoxic tumors of the head and neck are highly malignant and have high resistance to radiotherapy and chemotherapy. An assessment of hypoxia is important for selecting a suitable treatment method (eg hypoxia-targeting therapy), and to predict its efficacy. 18F-fluoroazomycin arabinoside (FAZA) positron emission tomography (PET) has been reported to be useful for non-invasive tumor hypoxic imaging, but it is not prevalent in clinical practice. Changes in cell structure caused by hypoxia could lead to altered pathways for water diffusion, which might be detected on diffusion-weighted magnetic resonance imaging (DWI). The purpose of this study was to investigate the usefulness of magnetic resonance imaging (MRI) as a hypoxia imaging tool by evaluating the correlation between DWI parameter estimates and FAZA-PET activity.Methods
Eleven patients with head and neck carcinoma (8 primary tumors and 5 lymph nodes without necrosis) were included in this retrospective study. DWI and FAZA-PET images were available as a part of a previous FAZA study1. The DWI was performed using a 3.0 T MRI system with b-values of 0, 50, 100, 200, 300, 500, 700, 900, 1100, 1300, 1500, 1700, 2000, 2300, 2600, and 3000 s/mm2. The parameters of the two-compartment model, the apparent diffusion coefficient (ADC) and the metrics of diffusion kurtosis imaging (DKI) were separately obtained from these images, pixel-by-pixel, as follows. To estimate the metrics of the 2-compartment model, the b-value dependent signal intensity change was fitted to the bi-exponential equation, Sb/S0 = Ffast*exp(-b*Dfast)+Fslow*exp(-b*Dslow), where b is the b-value, Sb/S0 is the normalized signal intensity at each b-value. The estimate parameters of this model are the diffusion coefficients of the fast and slow compartments (Dfast, Dslow) and the corresponding fractions (Ffast, Fslow). ADC based on mono-exponential fitting was also obtained at low (0 and 900 s/mm2) and high (1500 and 3000 s/mm2) b-value ranges (ADClowb, ADChighb). For the DKI, the image signal intensity for b-values from 200 to 2000 s/mm2 were fitted to the quadratic equation, Sb/S0 = exp(-b*D+1/6*b2*D2*K) to estimate the diffusional kurtosis (K) and the corrected diffusion coefficient (D). A region-of-interest (ROI) was manually drawn on each tumor in the slice where it had maximum diameter. The median values of the metrics inside the ROI were used for statistical analysis. For the FAZA-PET images, the tumor-muscle ratio (T/M = maximum standardized uptake value (SUV) of the tumor/mean SUV of the muscle) was measured in each case. The DWI estimates were statistically compared to the T/M of FAZA-PET using Spearman’s rank correlation coefficient. P-values under 0.05 were considered significant.Results
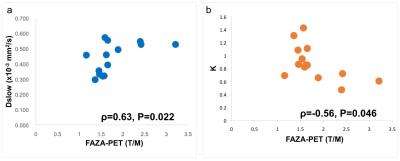
A significant positive correlation between Dslow and T/M ( ρ=0.63, P=0.022) and a negative correlation between K and T/M ( ρ=-0.56, P=0.046) were obtained (Fig. 1). There were no correlations between the other DWI parameters and T/M: Dfast ( ρ=0.31, P=0.30), Ffast ( ρ=-0.24, P=0.42), Fslow (ρ =0.24, P=0.42), ADClowb (ρ =0.42, P=0.16), ADChighb ( ρ=0.22, P=0.47), and D ( ρ=0.12, P=0.69).Discussion
The 2-compartment model divides the diffusing water molecules into fast and slow diffusion compartments. The slow compartment is thought to correspond to intracellular water molecules that interact highly with the cell membrane. Thus, destruction of cell membrane and increases in cell diameter may both contribute to elevate the diffusion coefficient of the slow compartment (Dslow). Decreased integrity of the cell membrane due to inflammation may also increase Dslow2. Those three factors (i.e. membrane destruction, increased permeability and increased cell diameter) have been previously reported in hypoxic tissue3. It is therefore possible that the significant correlation between Dslow and FAZA found in this study might reflect the increased permeability and destruction of the cell membrane due to hypoxia. However, the influence of cell diameter increases was probably small because Fslow did not correlate significantly with T/M. The ADC and two-compartment models implicitly assume that water diffusion is Gaussian. On the other hand, the diffusional kurtosis of the DKI model characterises deviation from Gaussian behaviour, so it is expected to provide another point of view with which to assess the complexity of tissue structure. Typically, K decreases under decreased complexity and reduced restriction in water diffusion4. The decrease of K in this study might also have reflected the increased permeability and destruction of cell membrane, but further studies will be needed to understand the detailed mechanism behind the results.Conclusion
The DWI parameters Dslow and K were significantly correlated with FAZA-PET activity, which suggests that they might be useful as indicators to assess tissue hypoxia.Acknowledgements
This work was supported by the grants from KAKENHI (15H04910), and Japan Advanced Molecular Imaging Program, MEXT, Japan.References
1. Saga T, Inubushi M, Koizumi M, et al. Prognostic value of PET/CT with (18)F-fluoroazomycin arabinoside for patients with head and neck squamous cell carcinomas receiving chemoradiotherapy. Ann Nucl Med. 2016;30(3):217-224.
2. Tachibana Y, Obata T, Yoshida M, et al. Analysis of normal-appearing white matter of multiple sclerosis by tensor-based two-compartment model of water diffusion. Eur Radiol. 2015;25(6):1701-1707.
3. Kumar V, Abbas AK, Fausto N, Mitchell RN. Robbins basic pathology 8th edition. Chapter 1. Cell injury, cell death, and adaptations. Philadelphia, PA: Saunders Elsevier; 2007.
4. Tachibana Y, Obata T, Tsuchiya H, et al. Diffusion-tensor-based method for robust and practical estimation of axial and radial diffusional kurtosis. Eur Radiol. 2016;26(8):2559-2566.
Figures