3459
Reproducibility of DTI Metrics and the Influence of SNR on DTI metrics in a Longitudinal Multicenter Clinical TrialXiaopeng Zhou1, Ken Sakaie1, Josef Debbins2, Robert Fox1, and Mark J. Lowe1
1The Cleveland Clinic, Cleveland, OH, United States, 2Barrow Neurological Institute, Phoenix, AZ, United States
Synopsis
SPRINT-MS is a large-scale longitudinal phase II trial including 27 3T MRI scanners to evaluate the treatment of progressive multiple sclerosis with Ibudilast. DTI measures showed high reproducibility in a longitudinal multicenter study, making it appropriate for use as a biomarker. The longitudinal design of the SPRINT-MS trial is expected to mitigate the systematic influence of SNR on DTI, but the observed trend may be useful as a correction factor in cross-sectional studies.
Introduction
High reproducibility is a key criterion for use of a DTI measure as a biomarker for disease progression and for the therapeutic effect of treatment. The inter-scanner variance of DTI metrics should also be incorporated when pooling data from different scanners. SPRINT-MS is a large-scale longitudinal phase II trial including 27 3T MRI scanners to evaluate the treatment of progressive multiple sclerosis with Ibudilast1. We evaluate scan-rescan reproducibility of DTI metrics and the influence of SNR on DTI metrics from scans acquired at each site in a longitudinal multicenter clinical trial.Methods
Scan-rescan images were acquired from 27 healthy volunteers aged 22 to 56 years (16 females, 11 males) on 27 MRI scanners (11 Siemens TIM Trio, 6 Siemens Skyra, 1 GE Signa EXCITE, 7 GE Signa HDxt, 1 GE DISCOVERY MR750 and 1 GE DISCOVERY MR750w) approved by a central IRB. Subjects were repositioned after the first scan, and the second scan was performed within one day of the first scan. Comparable HARDI sequences were acquired at each site with 2.5mm isotropic resolution with 64 b=700sec/mm2 and 8 b=0 volumes1. One site was limited to 55 diffusion-weighted volumes. All in vivo HARDI images were eddy current and motion corrected using Tortoise2. Pyramidal tracts identified with probabilistic tractography3. We evaluated the reproducibility of TD and FA along the pyramidal tracts. SNR along pyramidal tracts was calculated from the b = 0 images. Coefficient of variation (CV= 100 (SD /mean) %) was calculated to evaluate the reproducibility of each scanner. The dependence of TD and FA on SNR was evaluated with Pearson correlation. All calculation was performed using Matlab (MATLAB R2015a, The MathWorks Inc., Natick, MA 2015).Results
The CV values of TD and FA of each subject on each MR I scanner are less than 5%. The inter-scanner reproducibility was also examined by grouping different scanner models for scan1 and scan2 respectively (Fig.1). The CV of FA for scan1 and scan2 for each scanner model is less than 5.6%. The CV of TD for scan1 and scan2 for each scanner model is less than 5.0%. However, the CV of SNR for GE scan1 and scan2 are about 22.1%, for Skyra scan1 is 14.3%, for Trio scan1 is 10.3%. Although the CV of SNR for Trio scan2 (0.05%) and Skyra scan2 (8.8%) are much lower. The higher CV of SNR with GE scanner probably due to different head coils, GE scanners models with different software versions were grouped together. The dependence of DTI metrics on SNR was examined. There was a trend of increase in FA (R=0.60, p<0.0001) with SNR and decrease in TD (R=-0.63, p<0.0001) with SNR when SNR is less than 50 (Fig. 2).Discussion
Reproducibility across-session within each scanner and across scanners were evaluated in a longitudinal multicenter study. Measurements of DTI parameters can be affected by many factors in human subjects, such as the subject position, motion, auto-shimming before each sequence and biological variability. Overall, TD and FA showed good scan-rescan and inter-scanner reproducibility with 27 MRI scanners, which showed that DTI metrics are robust in this study. There is substantial variability of SNR across scanners with a significant effect on DTI measures. SNR may therefore serve as an easily-measured covariate when pooling data from different scanners. One limitation of this study is that only one subject was recruited at each site. There were no traveling subjects, making it difficult to differentiate contributions to variance from instrument and biological factors.Conclusion
DTI measures showed high reproducibility in a longitudinal multicenter study, making it appropriate for use as a biomarker. The longitudinal design of the SPRINT-MS trial is expected to mitigate the systematic influence of SNR on DTI, but the observed trend may be useful as a correction factor in cross-sectional studies.Acknowledgements
Supported by National Institutes of Health (U01NS082329 to Cleveland Clinic), National Multiple Sclerosis Society (RG-5184-A-6), Medicinova (through the National Institutes of Health), and individual grants from the National Institutes of Health to the Clinical Coordinating Center, Data Coordinating Center and each of the NeuroNEXT sites. The authors gratefully acknowledge technical support by Siemens Medical Solutions.References
1. Zhou X et al. . Magn Reson Imaging 2016; 35:81-90.
2. Pierpaoli C et al. Proc Intl Soc Mag Reson Med. 2010;18:1597.
3. Lowe M et al. Hum Brain Mapp 2008; 29(7):818-827
Figures

Fig.1 Across-session comparison of TD (left), FA (middle) and SNR (right) for GE, Trio and Skyra scanners. For each box, the central mark is the median, the edges of the box are the 25th and 75th percentiles, the whiskers extend to the most extreme data values not considered outliers, and the outlier points are plotted individually.
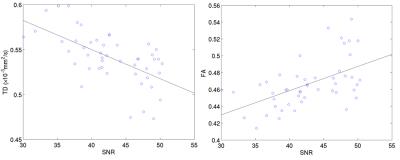
Fig.2 Dependence of TD (left) and FA (right) on SNR. The data points are from each DTI measure along each side of pyramidal tract with its corresponding SNR <50. The trend lines were added to show the correlation.