3451
Quantitation of DTI changes associated with muscle injury using a 3D printed phantom1Bioengineering, University of California, San Diego, San Diego, CA, United States, 2Nanoengineering, University of California, San Diego, San Diego, CA, United States, 3Radiology, University of California, San Diego, San Diego, CA, United States, 4Orthopaedic Surgery, University of California, San Diego, San Diego, CA, United States
Synopsis
Diffusion tensor imaging has been proposed as a tool to non-invasively assess skeletal muscle microstructure, which would be of significant clinical value. However, its application to the assessment of changes in muscle microstructure associated with injury, pathology, or age remain poorly defined because it is difficult to precisely control muscle microstructural features in vivo. Recent advances in bottom up fabrication technologies allow precision-engineered diffusion phantoms with histology informed skeletal muscle geometry to be manufactured. Therefore, the goal of this study was to develop skeletal muscle phantoms at relevant size scales in order to relate microstructural features to MRI-based diffusion measurements.
Introduction
Diffusion tensor imaging (DTI) has been used to measure changes in restricted diffusion in skeletal muscle after injury, which is thought to follow microstructural, and therefore, functional muscle changes. However, there are few direct comparisons between muscle microstructure and DTI measurements because it is difficult to precisely control in vivo experiments. Recent advances in bottom up fabrication technologies allow precision-engineered diffusion phantoms with histology informed skeletal muscle geometry to be manufactured. Therefore, the goal of this study was to develop skeletal muscle phantoms at relevant size scales in order to relate microstructural features to MRI-based diffusion measurements.Methods
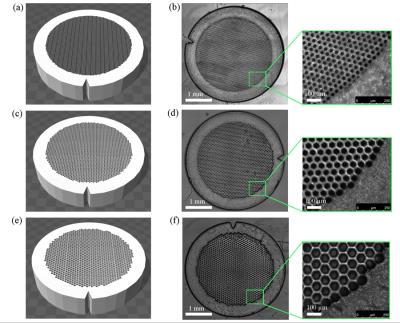
A digital light procession (DLP)-based 3D printing system was used to fabricate experimental phantoms (Fig. 1). Briefly, a collimated UV beam centered at 365nm wavelength illuminates the digital micromirror device (DMD), which contains an array of 2 million micromirrors, and is reflected toward the prepolymer solution. Digital masks are continuously loaded to the DMD chip to control the on/off state of each micromirror. The projection optics images the patterned UV light onto the prepolymer solution and polymerizes it as the pattern defined by the digital mask.
The prepolymer solution is a liquid state mixture of monomer or short-chain polymers, photoinitiators, and other additives. Polyethylene glycol diacrylate (PEGDA) was the main building material, lithium phenyl-2,4,6-trimethylbenzoylphosphinate was the photoinitiator, and TEMPO was the free radical quencher chosen for the phantoms. Upon stimulation by UV light, the photoinitiator generates free radicals locally. Then the free radicals attack the C=C bonds and generates acrylic monomers with free electrons, which react with the monomers and crosslink them into polymer networks. After exposure, the remaining liquid prepolymer is rinsed with PBS, and a solid structure remains.
As a first step, the complex geometry of skeletal muscle was simplified to closely packed hexagons in order to systematically study the relationship between muscle microstructure and MR-diffusion. Phantoms were printed in a 30μm, 50μm, or 70μm diameter hexagon array to approximate atrophic, normal, and hypertrophic human skeletal muscle respectively (Fig. 2). Additionally, to model increased extracellular water volume fraction associated with edema, the concentration of water in the 3D printed hydrogel was printed with 20% or 50% PBS. To demonstrate the ability to 3D-print geometrically complex phantoms, separate models were printed with histology informed geometry from normal rat muscle (tibialis anterior; TA) and an atrophic rat muscle (30-day denervated TA) (Fig. 3).
MR experiments were carried out using a 7T Bruker small animal imaging system with a 6cm gradient insert and a 300W quadrature transmit-receive volume coil. Samples first underwent a high-resolution 3D FLASH and then a series of diffusion weighted, spin-echo EPI sequences at different echo times (TE: 22.9ms, 19 echo times at 10ms interval, and additional echos at 253ms, and 303ms). The DT was calculated using the FanDTasia toolbox in Matlab [1].
Results
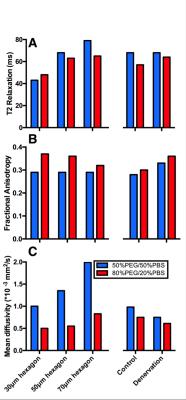
In general, the diffusion phantoms with 50% PBS had a higher T2 values than the phantoms with 20% PBS (Fig. 4A). Fractional anisotropy increased as the diameter of the hexagons decreased for the 20% PBS phantoms, but no difference was observed for the 50% PBS phantoms (Fig. 4B). Interestingly, mean diffusivity increased as fiber size increased for the 50% PBS phantoms, but no appreciable difference was observed between 30μm and 50μm diameter hexagons in the 20% PBS phantoms (Fig. 4C). When comparing the histology informed muscle geometry models, an increase in fractional anisotropy (Fig. 4B) and a decrease in mean diffusivity (Fig. 4C) was observed in the denervation-based sample compared to a control. However the magnitude of these changes was slightly smaller than what has been previously reported in the literature [2].Discussion
This study demonstrated a technique to fabricate phantoms with physiologically relevant geometries. These phantoms were then used to explore the validity of DTI as a method to measure microstructural changes in skeletal muscle. The range of diffusion and T2 relaxation values of the phantoms were similar to what has previously been found in muscle [3,4]; as fiber size decreased, a increase in the fractional anisotropy and a decrease in mean diffusivity was observed. Similarly, the differences in measured diffusion parameters between phantoms generated from normal and denervated muscle geometries is consistent with expected results from the literature and further supports the idea that DLP based 3D printing can be used to fabricate realistic skeletal muscle phantoms or constructs.Conclusion
This study demonstrates a novel application of tissue engineering to understand complex diffusion signals in skeletal muscle. Additionally, we have also demonstrated the feasibility 3D printing skeletal muscle with real-world matrix geometries and physiologically relevant tissue characteristics.Acknowledgements
No acknowledgement found.References
1. Barmpoutis, A. and B.C. Vemuri. A unified framework for estimating diffusion tensors of any order with symmetric positive-definite constraints. in 2010 IEEE international symposium on biomedical imaging: from nano to macro. 2010. IEEE.
2. Zhang, J., et al., Magnetic resonance imaging of mouse skeletal muscle to measure denervation atrophy. Experimental neurology, 2008. 212(2): p. 448-457.
3. Oudeman, J., et al., Techniques and applications of skeletal muscle diffusion tensor imaging: A review. Journal of Magnetic Resonance Imaging, 2015.
4. Esposito, A., et al., Magnetic resonance imaging at 7T reveals common events in age-related sarcopenia and in the homeostatic response to muscle sterile injury. PLoS One, 2013. 8(3): p. e59308.
Figures