3417
Detection of peripheral zone prostate cancer using diffusion-weighted intravoxel incoherent motion imaging1Department of radiology, the first affiliated hospital of medical college, Xi'an Jiaotong University, Xi'an, People's Republic of China
Synopsis
DWI has been proven to improve prostate cancer detection, and it reflects and measures the diffusion of water molecules within biological tissues due to thermal Brownian motion. However, to improve the significance of DWI, it is necessary to evaluate separately the two components of diffusion: the puremolecular diffusion and the perfusion-related diffusion originating from capillary microcirculation. IVIM-DWI, applying a bi-exponential fitting function, allows the extraction of pure molecular diffusion parameters (D) and perfusion-related diffusion parameters (D* and f). Therefore, the aim of this study is to further characterize and compare the biexponential nature of the diffusion related signal decay with multiple b-factors for PZ PCa and healthy PZ areas.
Introduction
Diffusion-weighted imaging (DWI) has been claimed to contribute to T2-weighted MRI in the detection and localization of PCa , particularly in the peripheral zone (PZ).1 To date, most DWI studies for the prostate have used b factors within the 0–2000 s/mm2 range, with ADC values calculated assuming monoexponential decay functions of signal with b value. In the intravoxel incoherent motion (IVIM) , the extravascular molecular diffusion and the microcirculation of blood within the capillaries can be separated using a biexponential decay function, providing additional parameters for tissue characterization.2 The objective of this study was to evaluate the diagnostic performance of the IVIM DWI model to differentiate between healthy tissue and PZ PCa.Methods
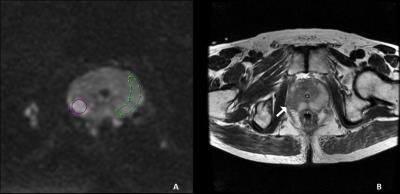
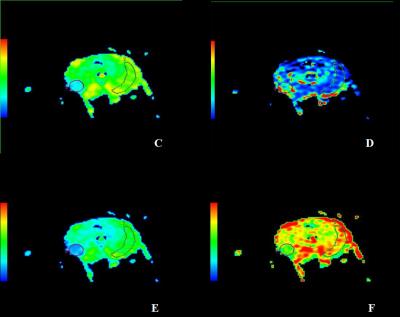
Our institutional review board approved this retrospective study and deemed that patient informed consent was not required. Patients 36 PZ PCa patients had obvious region of cancer on MRI and histopathology revealed cancer in the same region; normal-appearing contralateral PZ on MRI and no cancerous tissue revealed by histopathology in the corresponding PZ area; and no hemorrhage seen on T1-weighted images. MR Protocols All MR examinations were performed by a 3.0T MR scanner (GE, Signa HDxt, USA) using an eight-channel phased-array coil. T2WI was performed with TR/TE of 4720/107.5, 4mm slice thickness with 1mm gap, 512 × 512 matrices, and two excitations. IVIM DWI was performed with TR/TE of 6400/83.4, 4mm slice thickness with 1mm gap, 256×256matrices, and two excitations. The diffusion weighting was performed along three orthogonal directions at 11 b values of 0, 50, 100, 150,200,300,400, 500,800,1000 and 2000s/mm2. Data analysis Regions of interest were drawn in healthy and cancerous tissue of 36 patients with histologically proven PZ PCa and fitted to a monoexponential model [yielding the apparent diffusion coefficient (ADC)] and the IVIM signal equation (yielding the perfusion fraction f, the diffusion constant D and the pseudo diffusion coefficient of perfusion D*). Parameter maps were calculated for all parameters. Statistical analysis A paired t-test was performed to assess the statistical significance of the ADC, D, D* and f parameters between PZ cancerous and contralateral healthy tissues. A p value of <0.05 was considered as the threshold of statistical significance for paired t-tests.Results
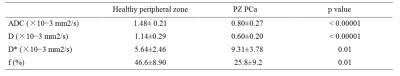
The characteristic signal decay and parameters were observed in the different tissues(Figs. 1-2 ). The mean values of ADC, D, D*, and f parameters measured in healthy PZ and PCa regions were 1.48± 0.21 vs. 0.80±0.27 (×10−3mm2/s, p < 0.00001), 1.14±0.29 vs. 0.60±0.20 (×10−3mm2/s, p < 0.00001), 5.64±2.46 vs. 9.31±3.78 (×10−3mm2/s, p = 0.01), and 46.6±8.90 vs. 25.8±9.2(%, p = 0.01), respectively (Table 1). We found that the values of ADC, D and f parameters were significantly reduced in the index lesion area compared to the ones of healthy tissues. The D* parameter was significantly increased in the index lesion area compare to the healthy PZ.Discussion
In the present study, we successfully performed IVIM DWI and found that signal decays from PZ andPZ cancerous showed biexponential behaviors, offering IVIM parameters of molecular diffusion coefficient, perfusion-related diffusion coefficient, and perfusion fraction. We also have presented here more-detailed measurements of IVIM DWI using 11 b values under 2000 s/mm2.3 The ADC、D values and f were significantly lower in the index lesion areas compared to those of the healthy PZ areas, and the result of f parameter is completely new compared with previous studies4-5. we also found a statistical significant difference of D* parameter calculated in the index area compared with healthy PZ. Similar results have been already published literature suggesting that cellular structural changes occur in premalignant and malignant lesions.6-7 These results underlie an important need to have appropriate parameters for tissue characterization updated.Conclusion
In conclusion, all of the IVIM parameters in PZ PCa were significantly difference than in noncancerous prostate tissue. Therefore, the biexponential parameters may offer new and more information pertaining to the tissue characterization in differentiating PZ PCa from benign prostate tissues.Acknowledgements
No acknowledgement found.References
1. J.C. Weinreb, J.O. Barentsz, P.L. Choyke, et al. PI-RADS Prostate Imaging-Reporting and Data System: 2015, Version 2, Eur Urol. (September) (2015).
2. R.V. Mulkern, A.S. Barnes, S.J. Haker, et al. Biexponential characterization of prostate tissue water diffusion decay curves over an extended b-factor range. Magn Reson Imaging. 2006; 24:563–568.
3. Shinmoto H, Oshio K, Tanimoto A, et al. Biexponential apparent diffusion coefficients in prostate cancer. Magn Reson Imaging. 2009; 27:355-359.
4. A. Lemke, F.B. Laun, M. Klau, et al. Differentiation of pancreas carcinoma from healthy pancreatic tissue using multiple b-values: comparison of apparent diffusion coefficient and intravoxel incoherent motion derived parameters. Invest Radiol. 2009; 44:769–775.
5. H.C. Thoeny, T. Binser, B. Roth, et al. Noninvasive assessment of acute ureteral obstruction with diffusion-weighted MR imaging: a prospective study. Radiology. 2009; 252:721–728.
6. H. Shinmoto, C. Tamura, S. Soga, et al. An intravoxel incoherent motion diffusion-weighted imaging study of prostate cancer. Am J Roentgenol. 2012; 4: 496–500.
7. S.F. Riches, K. Hawtin, E.M. Charles-Edwards, et al. Diffusion-weighted imaging of the prostate and rectal wall: comparison of biexponential and monoexponential modelled diffusion and associated perfusion coefficients. NMR Biomed. 2009; 22:318–325.
Figures