3414
Feasibility of Accelerated Simultaneous Multi-Slice Diffusion-weighted MR Imaging of the Prostate1Diagnostic and Interventional Radiology, University of Tuebingen, Tuebingen, Germany, 2Section on Experimental Radiology, University of Tuebingen, 3Diagnostic and Interventional Radiology, University of Tuebingen, 4University of Stuttgart, 5Department of Biomedical Magnetic Resonance, University of Tuebingen, 6Department of Urology, University of Tuebingen
Synopsis
Diffusion-weighted (DW) MRI of the prostate has increased the diagnostic accuracy for the detection of prostate cancer. However, acquisition time of DWI is still relatively long. Therefore, we evaluated the feasibility of simultaneous multi-slice (SMS) DWI for accelerated MRI of the prostate. Qualitative and quantitative image analyses in phantom, volunteer and patient measurements revealed similar image quality for DWISMS as compared to standard DWI sequences. Thus, DWISMS seems feasible for clinical routine in order to optimize patient throughput and economic efficiency, which is desirable, due to the recent implementation of prostate MRI into clinical guidelines and the expected increase in patient numbers.
Purpose
To
assess the feasibility of simultaneous multi-slice (SMS) single-shot
echo-planar-imaging (EPI) for accelerated diffusion-weighted imaging (DWI) of
the prostate in phantoms, healthy volunteers and patients. Material and Methods
For
phantom measurements a dedicated DWI phantom with different sucrose
concentrations (0-40%) was used. In addition, 10 healthy volunteers and
16 patients with clinically suspected prostate cancer were examined
for in-vivo measurements. All
examinations were performed on a 3T MRI system (Magnetom Skyra, Siemens
Healthineers, Germany). A prototype simultaneous multi-slice single shot
EPI sequence
(DW-EPISMS; Multi-band EPI package, Release 013a, Center for
Magnetic Resonance Research, University of Minnesota, USA, acquisition
time
3:14 min) was acquired using a SMS-acceleration factor of 2 and blipped
CAIPIRINHA with slice shift of FOV-phase/4. Additionally, a single-shot
EPI sequence
(DW-EPISS; acquisition time 6:12 min) was acquired serving as
standard of reference. All DWI measurements were performed with
monopolar
diffusion sensitizing gradients applied in three orthogonal directions
with
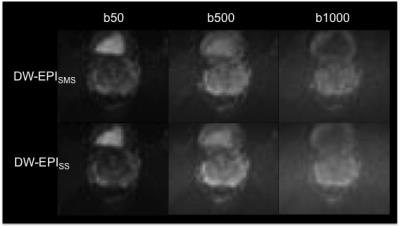
b-values of 50, 500, and 1000 s/mm2 and the following acquisition
parameters: repetition time (ms) 3000 and 6500 (DW-EPISMS and DW-EPISS
respectively); TE (ms) 62; acquisition matrix (mm) 220 x 108; resolution
(mm3)
2 x 2 x 3; and 6 averages. Image quality of DW-EPISMS was assessed
qualitatively (overall image quality, anatomic differentiability, lesion
conspicuity, image noise, distortion; two independent readers; 5 point
Likert-scale (5=excellent) and quantitatively (ADC-values, Coefficient
of
Variation (CV)) and compared to DW-EPISS. All statistical analyses
were performed using SPSS (Version 22, IBM, USA). Non-parametric data of
the
qualitative analysis were compared using Wilcoxon-signed-rank test.
Student´s
paired t-test was conduced for the comparison of normally distributed
data.Results
DW-EPISMS allowed for a significantly reduced acquisition
time as compared to DW-EPISS (≈50%). Overall image quality, anatomic
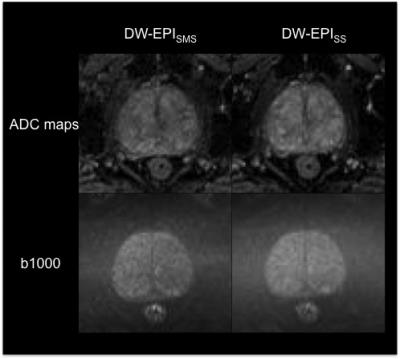
differentiability, lesion conspicuity and distortion of b1000 DW images and ADC
maps were rated similar for both sequences (p≥0.07; refer to Figure 1 and 2). Only in
the volunteer study, subjective image noise in DW images was rated
significantly higher in DW-EPISS than in DW-EPISMS (p=0.05,
see Figure 3). No significant differences were found for signal intensity of
b1000 DW images and ADC values (p≥0.07) between to two sequence types. Only CV
calculations of b1000 DW images revealed significantly higher values for DW-EPISMS
than for DW-EPISS (p<0.001).Discussion
In
this study we could demonstrate that DW-EPISMS is feasible for
prostate MRI allowing for substantially reduced acquisition time as compared to
DW-EPISS. We found similar image quality and signal intensity for
b1000 DW-EPISMS images as compared to the standard sequence indicating
the potential application of this technique in clinical routine. These results
are in line with several recently published studies evaluating the feasibility
of DW-EPISMS for DW imaging of the liver, also reporting similar or
even higher image quality as compared to DW-EPISS while
substantially reducing acquisition time1,2. Moreover, neither image
quality of ADC maps nor absolute ADC values revealed significant differences
between DW-EPISMS and DW-EPISS in both, phantom and in
vivo measurements. This is noteworthy, because adequate delineation of prostate
cancer on ADC maps is crucial for reliable measurements of ADC values, since
they have been reported to show a correlation with the Gleason score and may
reflect cancer aggressiveness3,4. These results are of
clinical interest, due to the recent implementation of prostate MRI into
clinical guidelines and the expected increase of patient numbers5. In this context, accelerated
DWI seems a helpful approach to optimize patient throughput and economic
efficiency.
Conclusion
Simultaneous multi-slice DWI is feasible for accelerated prostate MRI allowing for a substantially reduced examination time with similar image quality and ADC values as compared to a standard of reference DWI sequence.Acknowledgements
No acknowledgement found.References
1. Obele CC, Glielmi C, Ream J, et al. Simultaneous Multislice Accelerated Free-Breathing Diffusion-Weighted Imaging of the Liver at 3T. Abdom Imaging 2015;40(7):2323-2330
2. Taron J, Martirosian P, Schwenzer NF, et al. Scan time minimization in hepatic diffusion-weighted imaging: evaluation of the simultaneous multislice acceleration technique with different acceleration factors and gradient preparation schemes. Magma 2016;29(5):739-749
3. Luczynska E, Heinze-Paluchowska S, Domalik A, et al. The Utility of Diffusion Weighted Imaging (DWI) Using Apparent Diffusion Coefficient (ADC) Values in Discriminating Between Prostate Cancer and Normal Tissue. Pol J Radiol 2014;(79)450-455.
4. De Cobelli F, Ravelli S, Esposito A, et al. Apparent diffusion coefficient value and ratio as noninvasive potential biomarkers to predict prostate cancer grading: comparison with prostate biopsy and radical prostatectomy specimen. AJR Am J Roentgenol 2015;204(3):550-557.
5. National Collaborating Centre for C. National Institute for Health and Clinical Excellence: Guidance. Prostate Cancer: Diagnosis and Treatment. Cardiff (UK): National Collaborating Centre for Cancer (UK) Copyright (c) National Collaborating Centre for Cancer.; 2014.
Figures