3411
Distortionless diffusion weighted imaging of the prostate using a multi-shot Turbo Split Spin Echo BLADE sequence with bipolar diffusion gradientsBart WJ Philips1, Katharina Paul2, Thoralf Niendorf2, and Tom WJ Scheenen1
1Radiology and Nuclear Medicine, Radboud University Medical Centre Nijmegen, Nijmegen, Netherlands, 2Berlin Ultrahigh Field Facility (BUFF), Max-Delbrueck-Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany
Synopsis
Diffusion weighted imaging is an important modality in assessing and detecting prostate cancer, but often suffers from distortion artifact introduced by the EPI readout. We propose a TSE-BLADE split-echo sequence that has the potential for obtaining non-distorted diffusion weighted imaging of the prostate, while obtaining accurate ADC estimations. It is shown to be robust to motion and phase induced artifacts that pester multi-shot diffusion weighted imaging, in phantom measurements and initial patient results.
Introduction
Diffusion weighted imaging (DWI) is an essential part of the MRI protocol for detecting and assessing prostate cancer[1]. As DWI is generally based on EPI-like sequences, it suffers from geometrical distortions, leading to a mismatch with anatomical T2-weighted imaging. As a solution to the distortion problem, a diffusion sensitized Turbo Spin Echo (TSE)-based sequence can be used instead[2,3]. These approaches tend to show b-value dependent signal attenuations due to the motion sensitivity of the diffusion gradients, which leads to violations of the CPMG condition[4]. Additionally, the methods are multi-shot, introducing phase differences between k-space lines of different shots. We propose a multi-shot (ms)-TSE based sequence with bipolar diffusion gradients, a BLADE (Siemens proprietary name for PROPELLER) k-space trajectory and split-echo readout to counter the motion-induced artifacts of DW-TSE sequences in prostate MRI[5].Materials and methods
All measurements were performed on a 3T Prisma MRI system (Siemens Healthineers, Erlangen, Germany). A TSE-sequence with a multi-shot BLADE acquisition, was expanded with a diffusion preparation module using motion compensating bipolar gradients. An extra ADC event and readout gradient were added to each echo spacing to enable a split readout of the even (E1) and odd (E2) echoes[2,4] (Figure 1). The final image reconstruction added the E1 and E2 images in magnitude. Phantom measurements (container filled with iced water and 5 cylinders of demineralized water and one cylinder with a sucrose solution) were performed to evaluate the robustness of the sequence and its accuracy in determining the apparent diffusion coefficient (ADC). The TSE diffusion sequence was also run with a Cartesian readout (no BLADE), without split-echo readout and with monopolar diffusion gradients. As an additional test for robustness, motion-induced phase during diffusion gradients was simulated by varying the phase of the excitation pulse. Additional preliminary measurements were performed in one volunteer (26y) and a patient (74y) that underwent a prostate cancer detection examination. The sequence parameters for the patient measurement were chosen conservatively: TE=92ms, TR=4500ms, 10 slices, 3.0mm slice thickness, 128x128 matrix, FOV=320x320mm2, ETL=15, ESP=10.3ms, 26 blades and two b-values: 50 and 400s/mm2 (both acquired in 58s).Results
The phantom measurements showed that a 60° additional phase caused a severe signal attenuation in a TSE sequence without split-echo, due to destructive interference between odd and even echo groups caused by CPMG violations. The individual E1 and E2 echoes do not suffer from this loss and adding them in magnitude completely recovers the original signal intensity (Figure 2). This is also apparent from the ADC phantom measurements that show accurate ADC values with the split-echo TSE-sequence, whereas the TSE-sequence without split-echo yielded grossly overestimated ADC values in one ROI (Figure 3). The TSE split echo sequence with monopolar diffusion gradients yielded slightly less accurate ADC values, but also introduced artifacts that may have been caused by Eddy currents (Figure 3). In vivo results with Cartesian readout showed clear motion induced ghosting artifacts with monopolar diffusion gradients that were mitigated by using bipolar diffusion gradients (Figure 4). In vivo results with the TSE-BLADE split-echo sequence showed b0 and b400 images that were geometrically equal to the comparable T2W-image, whereas the EPI sequence showed some geometric distortion. Next to that, the ADC estimations of the TSE-BLADE sequence yielded accurate results (Figure 5).Discussion
The prostate is located within the abdomen and therefore suffers from motion, either caused by the prostate itself or by the abdominal wall or organs within the abdomen. The phantom measurements show the sensitivity of TSE-based sequences to motion induced phase that is introduced by adding diffusion gradients. The use of bipolar diffusion gradients can reduce Eddy currents and motion-induced effects. However, any residual motion-induced phase can still be severe and cause ADC inaccuracies. By using a split-echo approach this phase attenuation was mitigated. Motion-induced artifacts caused by phase differences between shots were further reduced by using a BLADE readout, as it inherently corrects for this[6]. The resulting sequence appears robust to these artifacts and yields diffusion weighted images with high geometric fidelity and accurate ADC estimations. A disadvantage of a split echo readout is the doubling of the readout time, resulting in some additional acquisition of noise. To take our research to the next level we will focus on improving SNR and on obtaining reliable image quality for high diffusion sensitization (b=800s/mm2) .Conclusion
The TSE-BLADE split-echo sequence shows potential for obtaining non-distorted diffusion weighted imaging of the prostate, while obtaining accurate ADC estimations. It proved to be robust to motion and phase induced artifacts that pester multi-shot diffusion weighted imaging, in phantom measurements and initial patient results.Acknowledgements
Siemens Healthineers for providing the TSE pulse sequence source code, Dutch Cancer Society [2014-6624]References
[1]Koh et al. AJR, 188.6(2007):1622-1635; [2] Paul et al.. Invest. Radiol. (2015); 50(5):309-321; [3] Zhang et al. Proc. ISMRM (2015), 0577; [4] Williams et al. MRM (1999): 1:734–742; [5] Deng et al. MRM (2008): 59:947–953; [6] Pipe. MRM (1999): 42(5):963-9.Figures
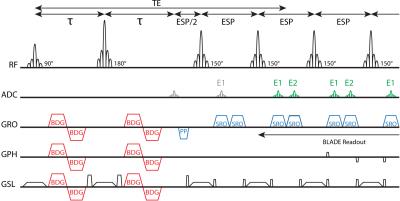
TSE
sequence with a bipolar diffusion gradient (BDG) preparation module, BLADE
readout and split echo readout (SRO). The prephaser (PP) gradient moment is
equal to 50% of one of the SRO gradients. In this case the RF pulses featured a
150° flip angle. The even echoes (E1) and odd echoes (E2) are depicted
schematically in the sequence diagram. The first E1 echo is skipped (gray) and acquisition starts during the next echo spacing when both E1 and E2 echoes are present (green).
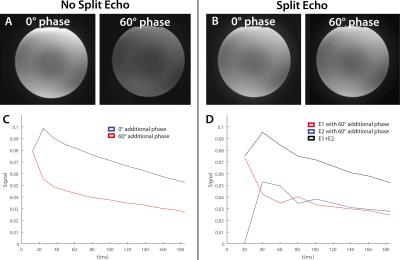
Images
of a water phantom obtained with the TSE-BLADE sequence without (A) and with
(B) split-echo readout. The excitation pulse was given an additional phase to
simulate motion during diffusion gradients. No diffusion gradients were used.
The signal of the echo-train (which was obtained in a separate measurement
without using phase-encoding gradients) was plotted for the sequence without (C)
and with split echo (D). The signal of the echo-train was plotted for the
different cases (C+D). (D) also shows the echo-train of the individual E1 and
E2 echoes and the magnitude addition of the two echoes. Flip angle = 150°.
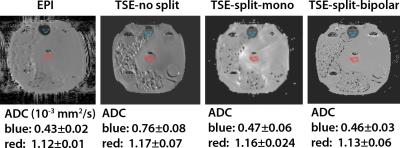
ADC images of a phantom with different
compartments are shown for an EPI diffusion sequence, TSE-BLADE without split
echo readout and bipolar diffusion gradients (TSE-no split), TSE with split
echo readout and monopolar diffusion gradients (TSE-split-mono) and TSE-BLADE with
split echo readout and bipolar diffusion gradients (TSE-split-bipolar). All ADC maps were reconstructed from b50, b400 and b800 images. The average ADC was
determined in two different ROIs (blue and red).
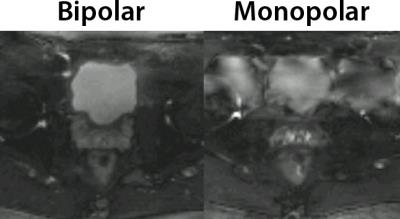
Two
images of the bladder and seminal vesicles obtained with a diffusion sensitized
TSE sequence without split echo and a Cartesian readout to illustrate the
effect of motion induce phase in multi-shot imaging. The left image is obtained
with bipolar diffusion gradients and the right with monopolar diffusion
gradients.

b50,
b400 and ADC images of a patient with the TSE-BLADE diffusion sequence with
split echo readout and with the standard DW-EPI sequence. As a comparison for
geometry the T2W image is shown. All images show the same slice. The red circle
indicates an ROI in the bladder in which the average ADC value was determined.