3406
Origin of diffusion anisotropy in human kidney: a combined DTI and IVM study1Faculty of Science and Technology, University of Twente, Enschede, Netherlands, 2Radiology, University Medical Centre Utrecht, Utrecht, Netherlands
Synopsis
To visualize the anisotropy of diffusion and pseudodiffusion, we have performed a two-tensor fit (D and D*) on IVIM data in healthy volunteers. We have performed tractography on both tensors and compared the results with tractography perfomed using a conventional DTI tensor fit. Tracking was possible using both D and the D* derived tensors and resulted in patterns similar to conventional tractography. We conclude that diffusion and pseudodiffusion both have an anisotropic component that can be visualized using tractography, which indicates that they both likely contribute to the coherent diffusion anisotropy that is detected in conventional monoexponential DTI.
PURPOSE
Diffusion MRI allows for the visualization and quantification of renal tissue and function without administration of exogenous contrast materials1,2. However, there is no consensus about the origin of anisotropy in the measured diffusion signal, as it can reflect multiple structures and active flow processes, such as the radial orientation of nephron tubules in the kidney medulla, perfusion and glomerular filtrate flow in the tubules. Hence, the purpose of this study was to study the anisotropy of different flow processes combining diffusion tensor imaging and intravoxel incoherent motion (IVIM) bi-exponential fitting.METHODS
Approval of our institution’s ethical committee was obtained for this research. Five healthy volunteers were scanned on a 3T Philips Ingenia clinical scanner. Subjects underwent T2 TSE imaging (TE: 100ms, TR: 2418ms, Matrix size: 400x320, FOV: 450x450mm2, voxel size: 1.13x1.41x3.0mm) as well as DTI (TE: 39ms, TR: 1267ms, Matrix size: 112x68, FOV: 336x204mm2, voxel size: 3.0x3.0x3.0, b-values: 0, 100, 300 s/mm2, gradient directions: 12) and IVIM imaging (TE: 45ms, TR: 1344ms, Matrix size: 112x68, FOV: 336x204mm2, voxel size: 3.0x3.0x3.0mm, b-values: 0, 10, 25, 40, 75, 100, 200, 300, 500, 700 s/mm2 gradient directions: 6). The DTI and IVIM acquisitions were respiratory triggered using a navigator. The diffusion tensor was calculated from the DTI data (“conventional DTI”) using ExploreDTI3 and D and D* were obtained from a two tensor fit using the IVIM scan with a custom-made MATLAB script. Whole kidney tractography was performed with DDTI, DIVIM and D*IVIM obtained tensors using ExploreDTI.RESULTS
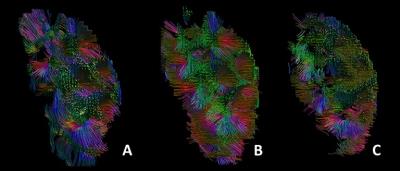
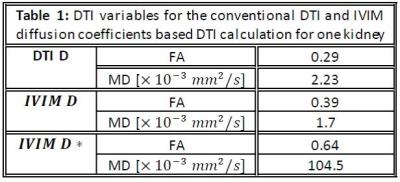
Tracking was possible using DIVIM and the D*IVIM derived tensors. Figure 1 shows the tractography results based on the tensor DTI (A) and two-tensor IVIM fit (B and C). The FA and MD for conventional DTI and IVIM based DTI calculation are displayed in table 1. The MD from DIVIM is lower than from DTI whereas the MD from D*IVIM is (about 50 times) higher. FA for DIVIM and D*IVIM are both higher than DTI derived FADISCUSSION
Previous studies have demonstrated the feasibility of DTI tractography in the kidney, resulting in radially oriented tracts that congregate into multiple papillae at the centre of the kidney1,2. Because these structures accord with the known microstructures of the kidney, i.e. tubules organised in multiple renal pyramids that terminate in papillae at the edge of the pyelum, it was concluded that diffusion tractography reflects tissue integrity of the renal medulla. An earlier combined DTI and IVIM study of the kidney showed that the pseudodiffusion component is also anisotropic.4 Here, we show that tractography is feasible based on the IVIM derived diffusion coefficient, associated with random diffusion, as well as the pseudodiffusion coefficient, associated with tissue perfusion. This suggests that the coherent diffusion anisotropy in the kidney that originates both in the radial organization of tubules in the renal pyramids and in perfusion by radially organized vasculature in the kidney medulla. An interesting point for further research is how these components each contribute to FA in conventional DTI: if the tracts from diffusion and pseudodiffusion DTI are aligned, the anisotropy from these components would strengthen each other, resulting in a higher DTI derived FA, whereas if they were dispersed, the IVIM derived anisotropies would counteract, resulting in a lower DTI derived FA. FA’s of the diffusion and pseudodiffusion components are each higher than the DTI derived FA, which suggests that the anisotropies are not aligned. However, differences in FA can also be the result of a lower SNR in diffusion and pseudodiffusion DTI compared to the total signal SNR. In addition, in the current tractography images, the exact orientations of the tracts cannot be verified.CONCLUSION
Diffusion and pseudodiffusion are both anisotropic which can be visualized using tractography. This indicates that they likely both contribute to the coherent diffusion anisotropy that is detected in conventional monoexponential DTI.Acknowledgements
No acknowledgement found.References
1 Chan RW, von Deuster C, Stoeck CT, et al. High-resolution diffusiontensor imaging of the human kidneys using a free-breathing, multislice,targeted field of view approach. NMR Biomed 2014;27:1300–1312.
2 Leemans et al. ExploreDTI: a graphical toolbox for processing, analyzing and visualizing diffusion MRI data. Proc 17th Annual Meeting ISMRM, Honolulu, 2009
3 Jaimes C, Darge K, Khrichenko D, Carson RH, Berman JL. Diffusion tensor imaging and tractography of the kidney in children: feasibility and preliminary experience. Pediatr Radiol; 44:30-41
4 Notohamiprojdo M, Chandarana H, Mikheev A, et al. Combined intravoxel incoherent motion and diffusion tensor imaging of renal diffusion and flow anisotropy. Magn Reson Med 2015; 73: 1526-1532