3398
Detection of distinct tract-specific axon diameter and density estimates in the in vivo human brain using TractCaliber MRI1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States, 2Computer Assisted Clinical Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 3Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
Synopsis
In this work, we apply the TractCaliber approach to a group of healthy subjects and show that distinct tract-specific estimates of axon diameter may be obtained in different white matter tracts in the normal human brain. Larger diameter axons are consistently estimated in the corticospinal tracts and are shown to be distinct from those in the cingulum, an adjacent and orthogonal white matter tract. The development of robust tract-specific axon diameter-weighted maps may be useful for refining existing tractography algorithms.
Purpose
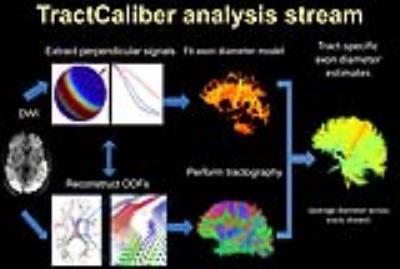
Diffusion tractography has been widely adopted as a tool for mapping structural connectivity between different brain regions in vivo. However, the accuracy of different tractography algorithms has been questioned, even with the use of high quality diffusion data (1). Complementing tractography with information on the underlying axonal microstructure may be helpful to achieve more quantitative and biologically plausible estimates of structural connections. We have recently introduced TractCaliber MRI, a method for axon diameter and density estimation across white matter tracts of arbitrary orientation in the living human brain (2). In this work, we apply the TractCaliber approach to a group of healthy subjects and show that distinct tract-specific estimates of axon diameter may be obtained in different white matter bundles in the normal human brain.Methods
Data acquisition: Twelve healthy volunteers (6M, 6F, ages 23-58) were scanned with approval from the institutional review board. All scans were performed on the dedicated high-gradient 3T CONNECTOM scanner with maximum gradient strength of 300 mT/m using a custom-made 64-channel head coil (3). To demonstrate intra-subject reproducibility, initial diffusion imaging protocols consisting of 1.5- and 2.4-mm3 resolution sagittal diffusion-weighted spin echo EPI images were acquired in separate scan sessions on a single healthy subject with whole brain coverage. The following parameters were used: TR/TE=3900/85 ms, diffusion gradient pulse duration δ=8 ms, diffusion times Δ=16/36/56 ms, 8 gradient strengths ranging from 30–290 mT/m per Δ for a total of 24 q-shells, 64 non-collinear diffusion-encoding gradient directions with 5 interspersed b=0 images, and parallel imaging (R=2). The total acquisition time for the 1.5- and 2.4-mm3 imaging protocols was 120 min and 103 min, respectively.
A shortened acquisition was then performed on all 12 healthy subjects. The protocol consisted of sagittal diffusion-weighted spin echo EPI images at 2-mm3 resolution throughout the whole brain, including the cerebellum and upper cervical spinal cord. The following parameters were used: TE/TR=77/3800 ms, δ=8 ms, Δ=19/49 ms, 8 gradient strengths ranging from 30–290 mT/m per Δ for a total of 16 q-shells, parallel imaging (R=2), and simultaneous multislice (MB factor=2). The diffusion gradients were applied in 64 non-collinear directions with 5 interspersed b=0 images for b-values greater than 2300 s/mm2. For b-values less than or equal to 2300 s/mm2, the diffusion gradients were applied in 32 non-collinear directions with 2 interspersed b=0 images. The maximum b-value at the longest diffusion time was 17,800 s/mm2. The total acquisition time for this imaging protocol was 55 min.
Data analysis: Following preprocessing to correct for distortions due to gradient nonlinearity, motion and eddy currents, the data were fitted using the TractCaliber approach outlined in Figure 1 to obtain axon diameter and volume fraction estimates (2). NODDI fitting was also performed using data with b=700 and 3100 s/mm2 to obtain estimates of orientation dispersion index (ODI) within white matter tracts. Combined volume and surface-based registration (CVS) in FreeSurfer (4) was used for cross-subject registration of axon diameter and volume fraction maps obtained from TractCaliber analysis.
Results
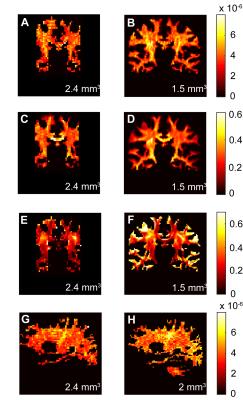
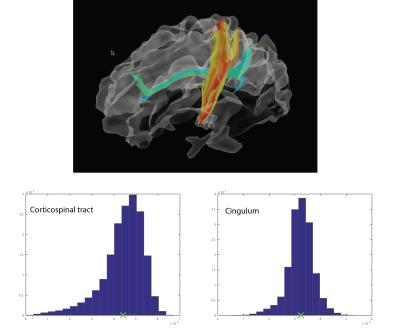
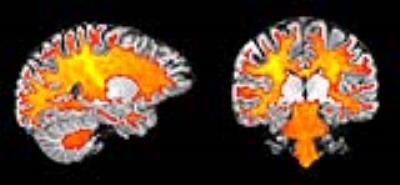
Figure 2 compares maps of axon diameter and density and ODI at different spatial resolutions for a single healthy subject. The axon diameter and volume fraction estimates were consistent for scans obtained at 1.5, 2 and 2.4 mm3 resolution and demonstrated larger diameter axons in the corticospinal tracts. Furthermore, the ODI maps showed relatively uniform orientation dispersion throughout the corticospinal tracts and other coherent white matter tracts, suggesting that dispersion was not a confounding factor in estimating axon diameter. Figure 3 shows that distinct tract-specific estimates of axon diameter and density could be obtained in the corticospinal tracts and cingulum in the same healthy subject. Figure 4 shows consistently larger estimates of axon diameter (~7 μm) in the corticospinal tracts compared to the adjacent white matter across all healthy subjects.Discussion
We demonstrate consistent tract-specific axon diameter and density estimates within and between multiple healthy subjects. The distinct and relatively uniform axon diameter estimates along the cingulum and corticospinal tracts agree with histological observations that axon diameters do not vary considerably along any given axonal projection (5). While the axon diameter estimates may not quantitatively match those obtained on histology, the larger axon diameters seen in the corticospinal tracts relative to the cingulum are in agreement with known histological trends (6).Conclusion
We demonstrate distinct tract-specific axon diameter estimates across white matter tracts of arbitrary orientation within and between healthy subjects, with trends that agree with those reported in the histological literature. The development of robust tract-specific axon diameter-weighted maps may be useful for refining existing tractography algorithms.Acknowledgements
Grant support: NIH NIBIB P41EB015896, U01MH093765, R00EB015445; NINDS K23NS096056, K23NS078044.References
1. Thomas C, Ye FQ, Irfanoglu MO, Modi P, Saleem KS, Leopold DA, et al. Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci U S A. 2014;111(46):16574-9.
2. Huang SY, Witzel T, Fan Q, McNab JA, Wald LL, Nummenmaa A. TractCaliber: Axon diameter estimation across white matter tracts in the in vivo human brain using 300 mT/m gradients. Proceedings of the 23rd Annual Meeting of the ISMRM., 2015. Toronto, Canada.
3. Setsompop K, Kimmlingen R, Eberlein E, Witzel T, Cohen-Adad J, McNab JA, et al. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. Neuroimage. 2013;80:220-33.
4. Postelnicu G, Zollei L, Fischl B. Combined volumetric and surface registration. IEEE Trans Med Imaging. 2009;28(4):508-22.
5. Perge JA, Koch K, Miller R, Sterling P, Balasubramanian V. How the optic nerve allocates space, energy capacity, and information. J Neurosci. 2009;29(24):7917-28.
6. Tomasi S, Caminiti R, Innocenti GM. Areal differences in diameter and length of corticofugal projections. Cereb Cortex. 2012;22(6):1463-72.
Figures