3394
Quantifying microstructure in low and high-grade brain tumours using VERDICT MRI1Centre for Advanced Biomedical Imaging, University College London, London, United Kingdom, 2Centre for Medical Imaging, Division of Medicine, University College London, London, United Kingdom, 3UCL Institute of Neurology, Department of Brain Repair and Rehabilitation, London, United Kingdom, 4Centre for Medical Image Computing, Department of Computer Science, University College London, London, United Kingdom, 5National Hospital for Neurology and Neurosurgery, London, United Kingdom, 6Division of Neuropathology, Institute of Neurology, University College London, United Kingdom
Synopsis
VERDICT (Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumours) MRI is a diffusion imaging technique which has shown promise in revealing information about tumour microstructure beyond ADC measures and structural imaging. We report the first application of VERDICT to human brain tumours. In this feasibility study, we characterise a mixed group of brain tumours using VERDICT, which includes a range of both low and high-grade gliomas; compare the VERDICT parameter maps with ADC maps, post-contrast T1w images and histological grading; and examine the repeatability of the technique.
Introduction
Gliomas are the most common primary malignant adult brain tumours. Glioblastoma (GBM) is the most aggressive subtype with a 5-year survival of less than 10%. For therapeutic trials in gliomas, traditional imaging predominantly focuses on the contrast-enhancing component of the tumour with only qualitative assessment of non-enhancing tumour1. With several therapeutic options now available for relapsed/recurrent disease, including vaccines and immunotherapy, better imaging assessment of tumour burden is needed. Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumours (VERDICT) is a diffusion MRI technique which is combined with mathematical modelling to non-invasively estimate and map microstructural tissue parameters such as cell density, radius and vascular perfusion2. Previously, VERDICT has been shown to differentiate malignant prostate cancer from benign pathology3. For the first time, we use VERDICT MRI to characterise both low and high-grade tumours. We apply a three-compartment model to characterise a range of gliomas, compare with histological grading and examine the repeatability of the technique.Methods
Study design: 9 untreated patients with brain tumours (3 glioblastoma (grade IV), 1 metastatic carcinoma (images not shown), 3 astrocytoma (II/III), 2 oligodendroglioma (II)) were scanned at 3T (Achieva, Philips) using an 8-channel head coil. Nine diffusion weightings were acquired (3-orthogonal directions, b = 80-3000 s/mm2) with a single-shot SE-EPI readout with the following parameters: TR = 3.7s, TE = min, FA = 90°, DM = 922, voxel size = 2.5mm3, slices = 33, averages = 1. A 15-direction DTI scan was also acquired (b = 700 s/mm2). Acquisition time was 12 minutes. DWIs were normalised to B0 (b = 0) images acquired with the same TE. Five patients underwent a second set of same-session scans for assessment of repeatability. For quantitative assessment, the repeatability coefficient was calculated for each parameter4.
Image processing and analysis: Diffusion images were registered and corrected for eddy current effects using the ECMOCO toolbox in SPM5. For VERDICT analysis, tumour masks were created by manual segmentation of B0 images. Voxel-wise fitting with VERDICT was conducted across the ROIs. A BallSphereStick model was used to estimate microstructural parameters including intracellular volume fraction (fic), extracellular v.f. (fees), vascular v.f (fv) and cell radius index. The diffusivities of each compartment were fixed for stability (dic = 1x10-9, dees = 2x10-9, dv = 8x10-9 m2/s). ADC maps were also generated for each patient. Mean parameter values were calculated for each parameter by averaging across the tumour ROI. Note, for GBMs, the peri-tumour zone was fitted but excluded from mean parameter calculations so that only bulk tumour regions were compared.
Results
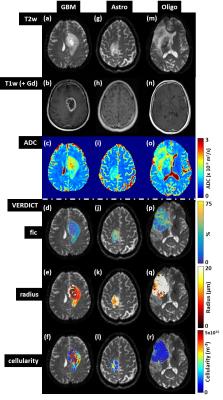
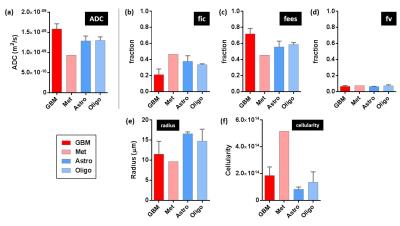
ADC values were elevated in tumour regions compared to normal brain for all gliomas types, as expected6 (Fig 1). ADC was highest in GBMs (Fig 2a). The astrocytoma and oligodendroglioma had comparable mean ADC values, whilst the metastatic carcinoma demonstrated the lowest ADC.
VERDICT parameter maps in the GBMs (Fig 2d-f) showed a rim around the core of the tumour where the fic, radius and cellularity (= fic/radius3) parameters were raised compared to the centre, consistent with a region of higher cell density surrounding a necrotic core. Interestingly, the parameter maps were non-uniform across the peri-tumour zone indicating variations in the underlying microstructure within this region. In the astrocytoma radius parameter and cellularity index maps (Fig 1j-l), there were areas of increased cellularity and reduced radius not seen as clearly in the corresponding structural image (Fig 1g) and ADC map (Fig 1i), which have a more uniform appearance. In the oligodendroglioma radius parameter map (Fig 1q), many voxels hit the upper fitting bound (of 20µm) suggesting that the modelling needs further adaptation for this tumour subtype.
Overall, the cellularity index was elevated in the grade IV GBMs (Fig 2f) compared to the grade II/III astrocytomas and oligodendrogliomas. The highest cellularity value was in the metastatic carcinoma. The VERDICT radius parameter was reduced in the GBMs compared to the astrocytomas and oligodendrogliomas (Fig 2e), however, this may be related to the fitting in the lower grade subtypes. Repeatability was generally excellent for all VERDICT parameters (Fig 3) with a repeatability coefficient <7% except for the cellularity parameter (15.5%).
Discussion
We have demonstrated that VERDICT is feasible in human gliomas and potentially provides information that corresponds to histological grading, which goes beyond ADC and conventional imaging. We are currently working on further validation of the VERDICT parameters with detailed histological analysis. Future work will focus on optimisation of the diffusion scans to permit shorter acquisition times for a clinically viable sequence that could potentially have important implications for pre-surgical planning and assessment of therapeutic response.Acknowledgements
No acknowledgement found.References
1PY. Wen, DR. Macdonald, DA. Reardon, et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. Journal of Clinical Oncology (2010).
2E. Panagiotaki, S. Walker-Samuel, B. Siow, et al. Noninvasive Quantification of Solid Tumor Microstructure Using VERDICT MRI. Cancer Research (2014).
3E. Panagiotaki, RW. Chan, N. Dikaios, et al. Microstructural characterization of normal and malignant human prostate tissue with vascular, extracellular, and restricted diffusion for cytometry in tumours magnetic resonance imaging. Invest. Radiol. (2015).
4JM. Bland & DG. Altman. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (1986).
5S. Mohammadi, HE. Moller, H. Kugel, et al. Correcting eddy current and motion effects by affine whole-brain registrations: evaluation of three-dimensional distortions and comparison with slicewise correction. MRM (2010).
6SE. Maier, Y. Sun & RV. Mulkern. Diffusion imaging of brain tumors. NMR in Biomed. (2010).
Figures