3376
A Novel Framework for Simulating the In-Vivo Diffusion MRI Signal in Solid Tumours, Based on High-Resolution Optical Imaging Data from Real-World Tumours.1Centre for Advanced Biomedical Imaging, University College London, London, United Kingdom, 2Mechanical Engineering, University College London, London, United Kingdom, 3Centre for Medical Image Computing and Computer Science, University College London, London, United Kingdom
Synopsis
We report the development of a novel in-silico modelling framework for probing the in-vivo diffusion MRI signal in tumours, based on high-resolution (5-10µm) optical imaging data from complete tumours. Blood flow in tissue substrates was estimated using fluid dynamical modelling. We then simulated the MRI signal using a Monte Carlo approach, and fitted the VERDICT model. VERDICT has previously been proposed as a method to noninvasively quantify histological features of tissue, including intracellular, extracellular and vascular volume fractions, cell radius and blood flow. We report preliminary findings of a good correspondence between the ground truth and measured values.
Purpose
The purpose of this study was to develop a realistic in-silico model of the diffusion MRI signal in tumour tissue, using three-dimensional, high-resolution imaging data from optical projection tomography of real-world tumours. This was in order to investigate the accuracy of the VERDICT model of compartmental diffusion, which has previously been proposed as a method to extract useful histologic features in tumours, such as cell radius, intra/extracellular volume fraction and vascular volume fraction1. However, validation of these measured features is a significant challenge due to the need for reliable ground truth values, which can be reliably adjusted within our substrate simulation platform.Methods
Simulation – Substrate Generation: The vessel structures for the tissue substrate (Figure 1) were generated from optical projection tomography data, acquired from a resected subcutaneous tumour (LS174T human colon adenocarcinoma), with vessels labelled by systemic administration of fluorescently conjugated lectin2,3. This allows complete, three-dimensional tumour blood vessels to be reconstructed, with a resolution of 5-10µm4. Fluorescent signal from vessels was segmented from background noise by subtracting a 3D Gaussian-filtered version of the data (25x25x25 kernel), then applying a Frangi filter to enhance vessel structures5. A skeletonisation algorithm was then applied, thresholded, and the result converted into graph format (nodes and segments). Blood flow values were estimated using a discrete network model. This model simulates Poiseuille flow through the individual segments with conservation of flux at network bifurcations. Unknown boundary conditions are estimated based on minimising the deviation of network pressures and wall shear stresses from specific target values6, and the model outputs flows and pressures throughout the network. Cells within the substrate were randomly generated using a sphere-packing algorithm, with a Gaussian distribution (mean 8µm, σ 0.1µm) of cell radii and the number of cells dictated by target intracellular volume fractions.
Simulation - Dynamics: Walkers were randomly initialised in the intracellular, extracellular or intravascular space within the substrate, with the number of walkers weighted by the volume fractions specified. The walker position was updated for each time-step of the simulation using a random-walk protocol7. At each step in the intra/extracellular space a new direction was chosen at random, and a step vector generated. The walker position was then updated, provided it did not cross a boundary between compartments (in which case the step is rejected). If the walker initialises in a vessel, the trajectory followed the direction and velocity of the flow within that vessel, given by the multi-scale modelling described above.
Signal Generation and Model Fitting: Diffusion-weighted MRI signal was generated from the recorded trajectories of the walkers using the Camino Diffusion MRI Toolkit8. A full 46 b-value, 3 direction VERDICT scheme was used to generate the signal in order to match preclinical data acquired previously. The ‘BallSphere’ and ‘BallSphereStick’ VERDICT models were then fitted to the ‘no-flow’ and ‘flow’ signals respectively, and the resulting parameters (intra/extracellular volume fractions, vascular volume fraction, cell radius) were compared against the ground truth values specified in the simulation to establish the correspondence between them.
Results
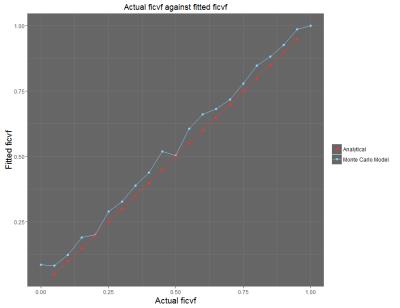
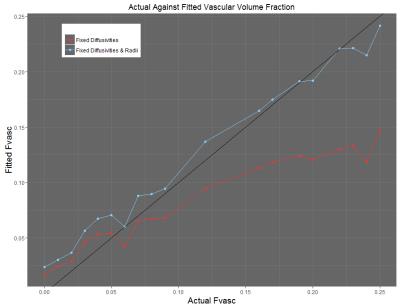
Initial results show that in the ex-vivo (no flow) case (Figure 2), the measured values of intracellular/extracellular volume fraction correspond closely with the ground truth values of the simulation. Preliminary results from the in-vivo case (flow in the vessels) show that the measured value of vascular volume fraction correlates with the ground truth value of the simulation, with a closer to 1-1 correspondence achieved when the cell radius value is fixed during the model fitting process (Figure 3). In both cases, other parameters such as the cell radius (when unfixed) also show close correspondence with the ground truth values provided by the simulation.Discussion and Conclusions
This study aimed to demonstrate an in-silico model of diffusion in tumour tissue as a potential tool for validating the mathematical models in the VERDICT MRI framework. The results discussed above show that generating the diffusion-weighted MR signal from an in-silico model of tissue provides a powerful tool for validating the mathematical models used to extract information about tissue microstructure. Figure 2 shows that the correlation of the parameters in the ‘no-flow’ case is very strong, indicating that the ‘BallSphere’ model is truly representative. The correlations shown in Figure 3 show that there is a degree of covariance between the vascular volume fraction and cell radius – though a strong correlation is seen regardless. One current limitation of this method is the simplistic nature of the representation of cells as spheres, and future work will aim to capture the chaotic and heterogeneous nature of the cellular structures found within tumour tissue.Acknowledgements
This work was supported by a Wellcome Trust Senior Research Fellowship (grant WT100247MA), KCL and UCL Comprehensive Cancer Imaging Centre CR-UK & EPSRC (In association with the DoH England), the EPSRC-funded UCL Centre for Doctoral Training in Medical Imaging (EP/L016478/1) and the Department of Health’s NIHR-funded Biomedical Research Centre at University College London Hospitals.References
1. Panagiotaki E, Walker-Samuel S, Siow B, et al. Noninvasive Quantification of Solid Tumour Microstructure Using VERDICT MRI. Cancer Research. 2014;74(7):1902-1912.
2. Oldham M, Sakhalkar H, Oliver T, et al. Three-dimensional imaging of xenograft tumours using optical computed and emission tomography. Med Phys. 2006;33(9):3193-3202.
3. D’Esposito A, Nikitichev D, Desjardins A, Walker-Samuel S, Lythgoe M.F. Quantification of Light Attenuation in Optically Cleared Mouse Brains. Journal of Biomedical Optics. 2015;20(8).
4. Kirbas C, Quek F. A Review of Vessel Extraction Techniques and Algorithms. ACM Computing Surveys (CSUR). 2004;36(2):81-121.
5. Frangi A, Niessen W, Vincken K, Viergever M. Multiscale vessel enhancement filtering. Medical Image Computing and Computer-Assisted Intervention. 1998;1496:130-137.
6. Fry BC, Lee J, Smith NP, Secomb TW. Estimation of Blood Flow Rates in Large Microvascular Networks. Microcirculation. 2012;19:530-538.
7. Hall M, Alexander D. Convergence and Parameter Choice for Monte-Carlo Simulations of Diffusion MRI. IEEE Transactions on Medical Imaging. 2009;28:1354-1364.
8. Cook P, Bai Y, Nedjati-Gilani S, Seunarine K, et al. Camino: Open-Source Diffusion-MRI Reconstruction and Processing. 14th Scientific Meeting ISMRM. 2006:2759.
Figures