3374
Microstructural alteration of amygdala-hippocampus-nucleus accumbens circuit in methamphetamine users : an in vivo diffusion tensor imaging study1Radiology, Lihuili Hospital, Ningbo, People's Republic of China, 2MR Research China, GE Healthcare, Shanghai, People's Republic of China, 3Ningbo Addiction Research and Treatment Center, Ningbo, People's Republic of China
Synopsis
No previous in vivo studies have investigated drug-related changes of microstructural integrity of amygdala-hippocampus-nucleus accumbens circuit using diffusion tensor imaging. This study demonstrated reduced microstructural integrity of this circuit in methamphetamine users by applying automated and semi-automated segmentation techniques to acquire regions of interest. The microstructural impairment of hippocampal subiculum and basolateral amygdala might be implicated in the development of anxiety and psychosis. Enhanced anatomical connectivity between left BLA and bilateral NAc may underlie the relapse of MA intake and production of psychosis.
Purpose
Among the brain regions involved in processing of drug rewards, the basolateral amygdala (BLA), hippocampal ventral subiculum (vSub) and nucleus accumbens (NAc) are strongly interconnected and form amygdala-hippocampus-NAc (AHN) circuit.1 This circuit has been implicated in inducing relapse in drug addiction, such as methamphetamine (MA), indicating that it may present structural abnormalities that underlie reinstatement of drug-seeking behavior. Animal studies have yielded evidence of alteration in this circuit.2 The volumetric changes of gray matter in MA users have been assessed on the level of macro-structure, which can hardly determine whether the volumetric enlargement is a result of inflammatory changes, and whether the volumetric reduction indicates a loss of neurons.3 Diffusion tensor imaging (DTI) measures provide a good opportunity to detect in vivo microstructural changes related to MA dependence by quantifying water diffusion behavior. In this study, we used DTI to investigate microstructural changes of AHN circuit in MA users.Methods
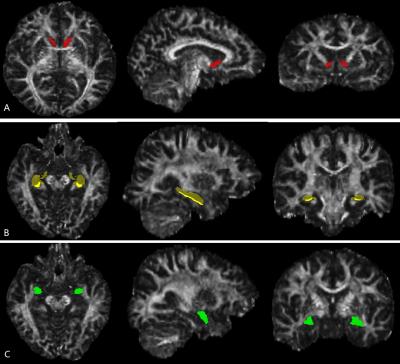
The study was approved by the local ethical committee and written informed consent was obtained from all the participants. Thirty male right-handed MA users (aged 34.9±7.3 years) were recruited according to the DSM-IV-TR criteria. The Brief Psychiatric Rating Scale (BPRS) score and its’ five factor scores, as well as the Hamilton Anxiety (HAMA) scores were assessed for MA users. Age- and gender-matched 30 right-handed healthy controls (aged 33.1±10.9 years) were recruited for group comparison. Three-dimensional fast spoiled gradient recalled echo (3D-FSPGR) and single-shot echo planar imaging acquisitions were performed to obtain structural T1WI and DTI data respectively using a 3.0-T MR750 scanner (GE Healthcare, Milwaukee, WI) with an 8-channel phase array head coil. The parcellation of NAc was performed using the fully automated Freesurfer tool (Figure 1). An improved pipeline in Freesurfer was applied for the segmentation of hippocampal subfields including subiculum. To acquire the segmentation of BLA, the probabilistic map of the BLA form a Jülich probability map at a threshold of 40% were transformed to structural space using FMRIB's Non-Linear Image Registration Tool (FNIRT). Fractional anisotropy (FA) and mean diffusivity (MD) maps were generated from raw DTI data using FMRIB's Diffusion Toolbox (FDT) in FSL, and were then transformed to structural space using FNIRT. The statistical analysis was repeated measures ANOVA to make comparison between MA and control groups with 1 between-subject factor (group: MA/controls) and 1 within-subject factors (hemisphere: left/right). Diffusion measures of AHN circuit were examined for Pearson’s partial correlations with duration of MA use, total dose of MA use, HAMA score, BPRS score and its’ five factor scores, with age as a nuisance covariate. Anatomical correlations between NAc and BLA/subiculum in MA/control group were also assessed using Pearson’s partial correlation analysis on diffusion measures of these structures.Results
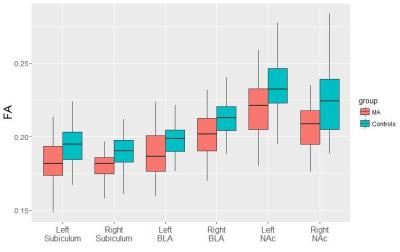
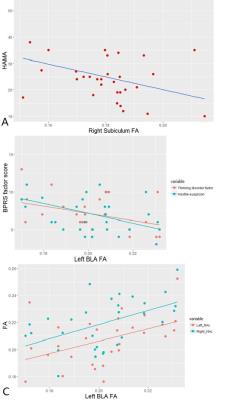
Compared with controls, MA users showed significantly lower FA in all 3 structures, i.e. BLA (F=10.414, p= 0.002), subiculum (F=5.719, p=0.02) and NAc (F=14.298, p=0.0004), with no significant group-by-hemisphere interaction, when the two hemispheres were included in repeated measures ANOVA (Figure 2). The higher total dose of MA use corresponded with lower FA in bilateral BLA (left, r=-0.622, p=0.0002; right, r=-0.649, p=0.0001), subiculum (left, r=-0.512, p=0.0039; right, r=-0.529, p=0.0027) and NAc (left, r=-0.525, p=0.0029; right, r=-0.494, p=0.0056). The HAMA score was negatively correlated with right subiculum FA (r= -0.377, p=0.040) (Figure 3). Lower left BLA FA was associated with higher thinking disorder factor score(r=-0.365, p=0.047), and hostile-suspicion factor score(r=-0.391, p=0.033) in BPRS (Figure 3). MA users presented significant association between left BLA FA and left/right NAc FA(left, r= 0.457, p= 0.011; right, r=0.51, p=0.004) (Figure 3).Discussion and Conclusion
MA users showed MA-induced impairment of microstructural integrity in all gray matter structures that constitute AHN circuit. Microstructural damage to subiculum may be implicated in weakening of its regulation on dopamine system, which would keep one in a constantly over-reacting status, i.e. anxiety. And microstructural impairment of BLA may play a key role in the development of psychosis. Enhanced anatomical connectivity between left BLA and bilateral NAc may underlie the relapse of MA intake and production of psychosis.Acknowledgements
No acknowledgement found.References
1. Grace AA. Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress. Neurotox Res. 2010;18(3-4):367-76.
2. Gill KM, Grace AA. Heterogeneous processing of amygdala and hippocampal inputs in the rostral and caudal subregions of the nucleus accumbens. Int J Neuropsychopharmacol. 2011;14(10): 1301-14.
3. Belujon P, Grace AA. Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Ann N Y Acad Sci. 2011;1216: 114-21.
Figures