3371
Diffusion - Tensor Based Morphometry (DTBM) of Normal Human Brain Development from Infancy to Adulthood1Quantitative Medical Imaging Section, National Institute of Biomedical imaging and Bioengineering, Bethesda, MD, United States, 2Section for Quantitative Imaging and Tissue Sciences, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, United States, 3Henry . M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, United States
Synopsis
During postnatal brain development brain structures undergo large changes in size, shape, composition and microstructural appearance. Diffusion tensor imaging (DTI) is an MRI modality particularly informative on white matter. We perform tensor based morphometry (TBM) using deformation fields constructed using all scalar and directional information provided by diffusion tensor data (DTBM) to measure volumetric changes of brain structures from neonate to adulthood. Our results indicate that DTBM reveals interesting patterns in the developmental trajectories for different structures in the human brain. This information would be important to characterize deviation from normal developmental patterns due to developmental delay or other disorders.
Purpose
During postnatal brain development brain structures undergo large changes in size, shape, composition and microstructural appearance. MRI is an important non-invasive technique that is valuable to characterize these changes. MRI images have been used to measure volume changes in white and grey matter with age.1 Tensor Based Morphometry (TBM), e.g. is a method that analyzes the Jacobian of the deformation necessary to align a brain to another brain (or a population template) to measure local differences in volume. Generally, TBM is performed from structural MRIs with relaxometry-based contrast, typically T1 weighted images (T1Ws). This may be problematic in the early stages of brain development where T1 and T2 values for brain structures are rapidly changing and there may be no differences in contrast between gray and white mater. Moreover, it is important to consider that even in the mature brain, the signal intensity within white matter appears relatively homogenous in T1Ws. Therefore it might be impossible to detect morphological changes of specific white matter pathways. Diffusion tensor imaging (DTI) 2,3 allows the identification of specific white matter pathways depicting the principal direction of diffusion that is locally collinear with the orientation of the fiber bundle. 4, 5 In a few cases TBM has been performed using diffusion MRI data 7,8,9,10 but in most works only scalar maps such as fractional anisotropy (FA) or low b-value DWIs were used to drive the registration. We hypothesize that TBM based on deformation fields obtained by registration of diffusion data (DTBM) will be informative in detecting morphometric changes in specific white matter pathways in the developing brain.Methods
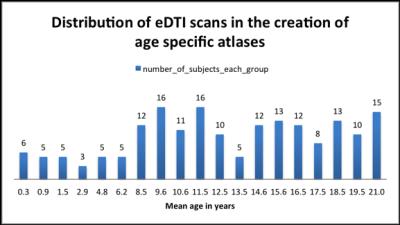
We included 182 subjects from the expanded DTI (eDTI) protocol of the publicly available database: the NIH MRI Normal Brain Development.11,12 We created 19 (Fig 1) age specific DTI average brain templates by co-registering the DTI data of individual subjects in a given age range using the recently proposed Diffeomorphic Registration for Tensor Accurate Alignment of Anatomical Structures (DR-TAMAS) algorithm.13 DR TAMAS uses all scalar and vectorial information contained in the diffusion tensor to achieve an accurate alignment of brain structures, with good performance in white matter (WM), gray matter (GM), and spaces containing cerebrospinal fluid (CSF). An “adult” template for the population was created from subjects in the 18-21yr old age group (mean age 19.5 years). Each age-specific template was then registered to the adult template using DR-TAMAS and the corresponding transformations (affine and nonlinear components) were used to compute voxelwise log of determinant of Jacobian (Log J) maps. Log J data were in turn used to compute the relative difference in volume between the age specific template and the adult template. The affine scaling value is obviously identical at each voxel location and accounts for global changes in brain volume across age. The non-linear component accounts for local volume differences in addition to those explained by global scaling. ROIs for various structures were drawn on the adult template and then average ROI values of Log J and FA were measured for each age specific template after morphing them into the adult template. ROI values of corresponding left and right structures were combined for analysis.Results and Discussion
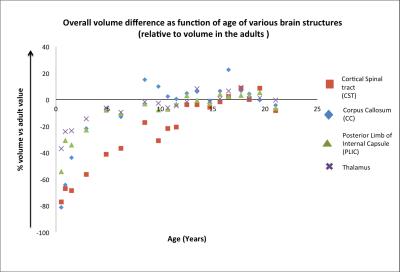
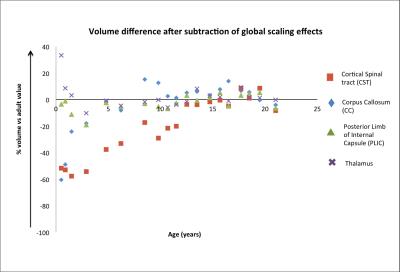
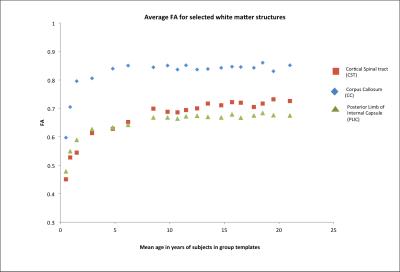
Fig 2 shows the overall volume difference (affine +non-linear component) as function of age of various brain structures, relative to their volume in the adults. For the same structures, Fig 3 shows the measured local changes in volume when the global scaling due to overall brain growth is subtracted. Both CC and CST at 0.5 years have a volume that need to increase by 80% to match their adult value. However CC increased in size rapidly during the first years of life reaching a plateau at 4 years, while CST showed a much slower pace reaching adult values at about 13 years. It is interesting to notice in Fig 3 that both CC and CST are growing at a rate much higher than the rest of the brain, while PLIC shows a growth trajectory, which is almost entirely explained by global volume changes. The Thalamus has indeed a growth rate in the early years that is slower than the rest of the brain (positive values in Fig 3). Finally, Fig 4 shows the measured values for FA with developmental trajectories quite different from those observed for the volumetric changes.Conclusion
Our results indicate that Diffusion MRI based TBM reveals interesting patterns in the developmental trajectories for different structures in the human brain. This information would be important to characterize deviation from normal developmental patterns due to developmental delay or other disorders.Acknowledgements
Support for this work included funding from the Congressionally Directed Medical Research Programs (CDMRP) (HJF Award#: W81XWH-13-2-0019).References
1. Giedd, JN ., et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb. Cortex. 6,(1996) 551-560.
2. Basser P.J, Mattiello J, and LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal (1994) 66:259-267.
3. Pierpaoli, C., Jezzard, P., Basser, P.J., et al. Diffusion tensor MR imaging of the human brain. Radiology (1996) 201:637-648.
4. Pajevic, S. and Pierpaoli, C. Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: application to white matter fiber tract mapping in the human brain. Magn Reson Med. (1999) 42:526-540.
5. Catani M, Thiebaut De Schotten M. Atlas of Human Brain Connections. Oxford: Oxford University Press, 2012. 456 p.
7. Pagani E, Agosta F, Rocca MA., et al. Voxel-based analysis derived from fractional anisotropy images of white matter volume changes with aging. Neuroimage. (2008) 41 (3): 657-667.
8. Oishi K, Faria A, Jiang H., et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. Neuroimage (2009) 46(2): 486–499.
9. Verma R, Mori S, Shen D., et al. Spatiotemporal maturation patterns of murine brain quantified by diffusion tensor MRI and deformation-based morphometry. Proc. Natl. Acad. Sci. U. S. A., 102 (2005), pp. 6978–6983.
10. Sadeghi N, Thomas CP, Irfanoglu MO., et al. "Deformation Analysis of Diffusion Tensor Data: Application to Hereditary Spastic Paraplegia." In ISMRM 24th Annual Meeting and Exhibition, Singapore. May 2016.
11. Walker L, Chang LC, Nayak A ., et al. The diffusion tensor imaging (DTI) component of the NIH MRI study of normal brain development (PedsDTI). Neuroimage (2016)124, 1125–1130.
12. NIH MRI Study of Normal Brain Development, http://pediatricmri.nih.gov .
13. Irfanoglu MO, Nayak A, Jenkins J., et al. DR-TAMAS: Diffeomorphic Registration for Tensor Accurate Alignment of Anatomical Structures. Neuroimage (2016) 132:439–454.