3366
Influence of post-processing method on the repeatability of diffusion-weighted imaging parameters in healthy brain1Translational Bioimaging Group, Barrow Neurological Institute, Phoenix, AZ, United States, 2Imaging Programs, National Comprehensive Cancer Network (NCCN), Philadelphia, PA, United States, 3University of Texas - Austin
Synopsis
The purpose of this study is to investigate the influence of post-processing method on the reproducibility of brain diffusion metrics, including apparent diffusion coefficients (ADCs) and intra-voxel incoherent motion (IVIM) parameters, in healthy controls and to apply these results in a cohort of brain tumor patients undergoing treatment. ADC was highly reproducible for all methods. The IVIM diffusion and perfusion fraction showed the highest reproducibility using constrained fitting, while IVIM pseudo-diffusion showed limited reproducibility. By establishing limits of repeatability for ADC and IVIM metrics, these methods can be applied in neuropathology to determine significant changes related to treatment effects.
Introduction
Diffusion-weighted imaging (DWI) is a commonly employed biomarker of cellular status in in neuro-oncology. For example, changes in the apparent [water] diffusion coefficient (ADCs) reflect treatment-induced tumor cellular changes.1 However, a critical obstacle for the development of diffusion-based biomarkers comes in differentiating between a true treatment effect and confounding measurement inaccuracies.2 Using a standard post-processing method, both ADC and the intra-voxel incoherent motion (IVIM) diffusion coefficient D were shown to be highly reproducible across scanners, while the IVIM perfusion fraction fp showed moderate reproducibility.3 As the choice of post-processing method also affects the reliable derivation of diffusion metrics,4 the purpose of this study is (i) to investigate the influence of post-processing method on the reproducibility of brain diffusion metrics in healthy controls and (ii) to apply these results in a cohort of brain tumor patients undergoing treatment.Methods
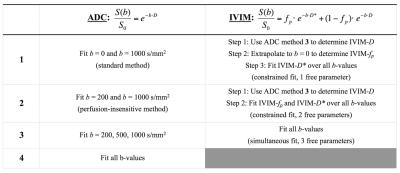
Data were acquired at 3T (Achieva, Philips Healthcare) in healthy controls (n = 9) and high-grade glioma patients (n = 6) at three time points (time 0, 1 week, and 4 weeks). The patient data corresponded with before and 2 time points after Bevacizumab treatment. Images were acquired with a single-shot spin-echo EPI sequence with TR = 6 s, TE = 50 ms, SENSE parallel imaging (2 in AP direction), voxel size = 2.5 mm isotropic, 40 slices, with three orthogonal diffusion-encoding directions and 8 b-values (b = 0, 25, 50, 75, 100, 200, 500, 1000 s/mm2). All data were registered to the T1-weighted image obtained at the 2nd time point using the affine (12 DOF) registration algorithm FLIRT (FSL, FMRIB Centre, Univ. of Oxford). The segmentation algorithm FAST (FSL) was used to segment white matter (WM) and gray matter (GM) regions of interest (ROIs). The diffusion data were normalized to the b = 0 data (S0) and then fit to mono-exponential and bi-exponential equations to obtain ADC and IVIM parameters. For ADC, four post-processing methods were compared, while three post-processing methods were compared for IVIM (equations and methods are listed in Table 1). Repeatability was assessed using previously published methods.5Results / Discussion
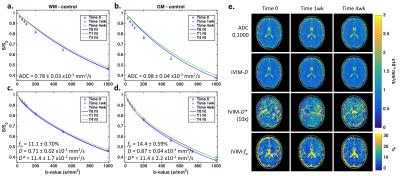
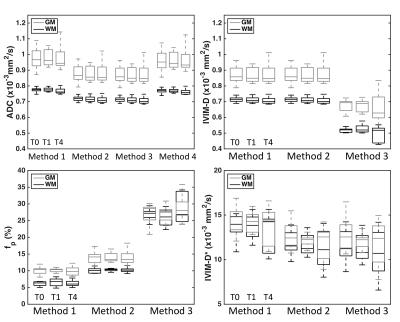
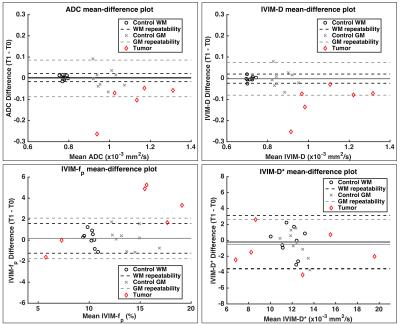
Figure 1 (a-d) shows DWI data and Method 1 fits for ADC (a,b) and IVIM (c,d) parameters in healthy control WM (a,c) and GM (b,d) ROIs over three time points. Signal from each b-value is consistent between time-points, yielding consistent ADC and IVIM-D maps (Figure 1, e). Visually, IVIM-D* did not appear reproducible across time points, while IVIM-fp appeared more consistent. Boxplots (Figure 2) for all patients and time points demonstrate the influence of post-processing methods on ADC and IVIM metrics. Across post-processing methods, the highest coefficient of variation (CV) for ADC was observed for Method 1 (1.99%), though not statistically different from Method 2-4 CV (1.86% for all). It should also be noted that the ADC values for Method 1 and 4 are higher due to increased sensitivity to perfusion effects at low b-values.6 For IVIM, Method 1 yielded the highest variability (CV = 5.5%, 8.1%, and 13% for IVIM-D, IVIM-fp, and IVIM-D*, respectively). IVIM-fp had the lowest CV (4.6%) for Method 2. Overall, IVIM-D* was not consistently fit by any method. Figure 3 shows Bland-Altman mean-difference plots between T0 and T1 (1-week) in control WM, control GM, and tumor ROIs using Method 1 ADC and Method 2 IVIM parameters. The repeatability limits were less than ±0.1x10-3 mm2/s for ADC and IVIM-D and less than ±2% for IVIM-fp. IVIM-D* showed wider limits of repeatability (< ±4x10-3 mm2/s). Using these limits applied to the patient cohort, several individual tumors showed 1-week tumor diffusion changes outside of the repeatability limits. Statistical analysis using repeated measures analysis of variance is ongoing.Conclusions
We have demonstrated the influence of post-processing methods on the derived diffusion metrics ADC, IVIM-D, IVIM-fp, and IVIM-D*. All four tested methods for ADC showed similar variance, though methods that include data from b = 0 s/mm2 are sensitive to perfusion effects that may affect the resulting ADC values. Simultaneous fitting for all IVIM parameters is not recommended due to the high variability in the results parameters. IVIM-fp can be reproducibly measured using either constrained fitting method, but IVIM-D* is not reproducible. This study has permitted us to establish expected levels of variability in ADC and IVIM metrics over time, and variations outside of these limits may indicate altered pathology related to tumor growth or treatment effects.Acknowledgements
This work was supported by NIH/NCI 1R01CA158079, NIH/NCI U01 CA142565, NIH/NCATS KL2 TR 000446, and NIH/NCATS RR024975.References
1. Chenevert TL, Stegman LD, Taylor JMG, et al. Diffusion Magnetic Resonance Imaging: an Early Surrogate Marker of Therapeutic Efficacy in Brain Tumors. Journal of the National Cancer Institute 2000;92(24):2029-2036.
2. Padhani AR, Liu G, Mu-Koh D, et al. Diffusion-Weighted Magnetic Resonance Imaging as a Cancer Biomarker: Consensus and Recommendations. Neoplasia 2009;11(2):102-125.
3. Grech-Sollars M, Hales PW, Miyazaki K, et al. Multi-centre reproducibility of diffusion MRI parameters for clinical sequences in the brain. Nmr Biomed 2015;28(4):468-485.
4. Meeus EM, Novak J, Withey SB, Zarinabad N, Dehghani H, Peet AC. Evaluation of intravoxel incoherent motion fitting methods in low-perfused tissue. Journal of Magnetic Resonance Imaging 2016.
5. Galbraith SM, Lodge MA, Taylor NJ, et al. Reproducibility of dynamic contrast-enhanced MRI in human muscle and tumours: comparison of quantitative and semi-quantitative analysis. Nmr Biomed 2002;15(2):132-142.
6. Cohen AD, LaViolette PS, Prah M, et al. Effects of perfusion on diffusion changes in human brain tumors. Journal of magnetic resonance imaging : JMRI 2013;38(4):868-875.
Figures