3352
Evaluation of diffusion directions vs averages for accurate and reproducible cardiac DTI1Cardiovascular Medicine, University of Oxford, Oxford, United Kingdom
Synopsis
Cardiac DTI is subject to long scan times and low SNR, which invariably leads to a trade-off between the number of averages and diffusion-encoding directions to acquire. However, the exact relationship between the diffusion tensor and these parameters is unclear. In this work we utilise DTI data from five high quality ex-vivo rat hearts, and vary the SNR between 2 and 97 and the number of directions between 7 and 61. Results show that the apparent diffusion coefficient is optimised for scan time when SNR is maximised and directions are minimised, whereas parametric angle measurements are time-optimised with more directions. At typical in-vivo settings, we estimate that fractional anisotropy is being overestimated by up to 20%, while the precision of sheetlet angles may be as poor as ±36 degrees.
Purpose
Diffusion tensor imaging (DTI) minimally requires six diffusion-weighted (DW) images and fitting a diffusion tensor to each voxel. In cardiac DTI, the principal eigenvectors of this tensor, v1,2,3, may be ascribed to the locally prevailing cell long-axis, sheetlet, and sheetlet-normal directions respectively. However, reliable sorting of eigenvectors in the presence of noise, and therefore reliable estimation of sheetlet angles, remains a challenge1.
Invariably, a trade-off must be made between the number of DW directions and the number of averages that affects the signal-to-noise ratio (SNR). In this work we degrade high quality DTI datasets of ex vivo rat hearts in terms of numbers of DW directions and SNR, to establish their effect on the accuracy and precision of DTI parameters. This evaluation is performed in the absence of confounding factors such as strain and motion. Although the analysis is performed on ex-vivo samples, the results will translate to the in-vivo setting, which is subject to similar compromises.
Methods
Five hearts were excised from female Sprague-Dawley rats and imaged on a 9.4T preclinical MRI scanner as described previously2. Sixty-one DW directions were specified3, wherein the encoding scheme may be truncated while maintaining an approximately uniform spherical distribution. Four non-DW images were also acquired. The images were low-pass filtered with a 2nd order Butterworth filter to reduce Gibbs ringing and to increase SNR to 97.
Complex white noise was added independently to the real and imaginary channels of the DW images for SNR levels between 2 and 97, where the signal is given by the mean tissue signal intensity in the non-DW images. Diffusion tensors were derived by subsampling the number of directions from between 7 and 61. Ground truth tensors were derived from all 61 DW-images with no added noise. All tensors were computed using linear least squares. The parameters derived from the tensor were the eigenvalues λ1-3, mean apparent diffusion coefficient (ADC), fractional anisotropy (FA), helix angle (HA), transverse angle (TA), sheetlet elevation (SE) and sheetlet azimuth (SA), as defined by Teh et al.2 Accuracy and precision of parameters were computed from the mean and standard deviation of the difference between the estimated parameters and the ground truth.
Results
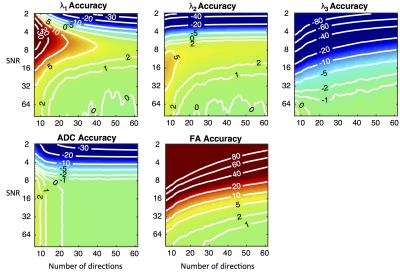
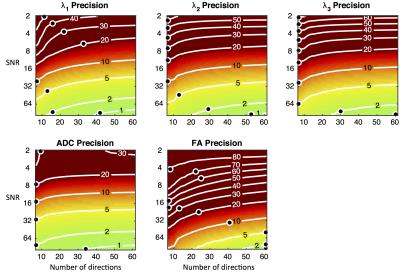
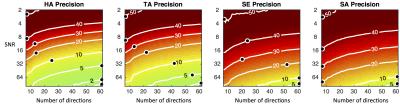
Figures 1 and 2 present the accuracy and precision of the principal eigenvalues, mean ADC, and FA. Figure 3 presents the precision of the parametric angles. For SNR > 6 and over a wide range of DW directions, our results indicate an over-estimation of λ1 and λ2, and under-estimation of λ3, leading to over-estimation of FA. The precision of ADC is time-optimised with a minimum of diffusion directions, and given a minimum SNR of 8, the precision of the HA and TA is time-optimised with more than six directions. The accuracy of the four angle maps (not shown) was within ±1%, regardless of SNR or number of DW directions. Figure 4 presents a comparison of the ground truth parametric maps with those derived from degraded data with typical in-vivo parameters.Discussion
At currently reported in-vivo human DTI settings, (i.e. 6-10 directions, SNR = 26-34 after averaging)3-5, FA may be over-estimated by 8-20%. This may be one contributing factor to the observation that ex-vivo studies2, with higher SNR and more DW directions, often yield lower FA estimates than in-vivo studies. The superior precision of HA and TA over SE and SA is due to the similarity in magnitude of λ2 and λ3. At in-vivo settings, we estimate that HA, TA, SE, and SA have precisions of 13-20, 17-23, 31-36 and 29-34 degrees respectively.
This study was limited to the analysis of a single shell scheme with a b-value of 1000 s/mm2. This b-value has been shown to minimise bias and absolute error in cardiac DTI parameters4. Furthermore, the resolution employed in this study is far higher to that of in-vivo acquisitions. Acquisitions at a lower resolution will likely yield lower FA values as a result of increased cell dispersion.
Conclusion
The number of DW directions and number of averages in cardiac diffusion MR experiments are important imaging parameters, which have non-linear impacts on the diffusion tensor. Accurate and precise estimation of sheetlet angles is more demanding than estimating HA and TA, which in turn is more demanding than estimating mean ADC. Our results indicate that with current in vivo imaging protocols, the precision of sheetlet angles may be as poor as ±36°. While this evaluation was performed in ex vivo heart, the findings are generalizable to the in vivo setting that is subject to similar compromises.Acknowledgements
This work was supported by the British Heart Foundation (BHF) [grant numbers PG/13/33/30210, RG/13/8/30266, FS/11/50/29038, FS/12/17/29532 and NH/13/30238], the Engineering and Physical Sciences Research Council [grant number EP/J013250/1], and the BHF Centre for Research Excellence [grant number RE/13/1/30181. The authors acknowledge a Wellcome Trust Core Award [grant number 090532/Z/09/Z]; and the European Research Council Advanced Grant [CardioNECT].References
1. Bernus O, Radjenovic A, Trew ML, LeGrice IJ, Sands GB, Magee DR, Smaill BH, Gilbert SH. Comparison of diffusion tensor imaging by cardiovascular magnetic resonance and gadolinium enhanced 3D image intensity approaches to investigation of structural anisotropy in explanted rat hearts. J Cardiovasc Magn Reson 2015;17:31.
2. Teh I, McClymont D, Burton RA, Maguire ML, Whittington HJ, Lygate CA, Kohl P, Schneider JE. Resolving Fine Cardiac Structures in Rats with High-Resolution Diffusion Tensor Imaging. Sci Rep 2016;6:30573.
3. Nielles-Vallespin S, Mekkaoui C, Gatehouse P, Reese TG, Keegan J, Ferreira PF, Collins S, Speier P, Feiweier T, de Silva R and others. In vivo diffusion tensor MRI of the human heart: reproducibility of breath-hold and navigator-based approaches. Magn Reson Med 2013;70(2):454-65.
4. Scott AD, Nielles-Vallespin S, Ferreira PF, McGill LA, Pennell DJ, Firmin DN. The effects of noise in cardiac diffusion tensor imaging and the benefits of averaging complex data. NMR Biomed 2016;29(5):588-99.
5. von Deuster C, Stoeck CT, Genet M, Atkinson D, Kozerke S. Spin echo versus stimulated echo diffusion tensor imaging of the in vivo human heart. Magn Reson Med 2016;76(3):862-72.
Figures