3344
Simultaneous Multi-Slice Double Diffusion Encoding Imaging1Electrical Engineering, Stanford University, Stanford, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States, 3Center for Cognitive and Neurobiological Imaging, Stanford University, CA, United States
Synopsis
Double diffusion encoding (DDE) sequences can measure microscopic diffusion anisotropy even in complex structures such as cortex or crossing fiber regions in white matter. However, DDE sequences require long TEs to accommodate the additional diffusion gradients, resulting in lower signal-to-noise ratio (SNR) and reduced slice coverage limiting its clinical feasibility. In this work, we implement a DDE sequence with simultaneous multi-slice (SMS) to improve the SNR efficiency and allow for full-brain DDE scans in a clinically feasible time.
Introduction
Double diffusion encoding (DDE) MRI1-6 encodes diffusion twice, along two different orientations, before the readout and has the potential to map the shape (eccentricity) of tissue compartments (known as “microscopic anisotropy”) even when the net “macroscopic” diffusion is isotropic. In this way, the fractional eccentricity (FE)2 measured by DDE is more specific to fiber integrity than fractional anisotropy (FA). However, DDE sequences require long TEs to accommodate the additional diffusion gradients, resulting in lower signal-to-noise ratio (SNR). In this work, we implement a DDE sequence with simultaneous multi-slice7 (SMS) to improve the SNR efficiency and allow for DDE scans with full-brain coverage and 2.5 mm isotropic resolution, in a clinically feasible time.Methods
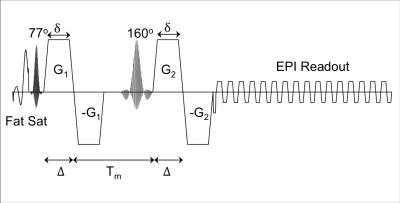
Data Acquisition: IRB approved experiments were conducted on a healthy subject using a 3T whole-body MR system (MR750, GE Healthcare, Madison, Wisconsin; 50 mT/m, 200 mT/m/s gradients) equipped with a 32-channel phased array head coil (Nova Medical). DDE images were obtained with a SMS single-SE-EPI pulse sequence with bipolar diffusion gradients on either side of the refocusing pulse shown in Figure 1. A variable rate excitation8 (VERSE) refocusing pulse is used for SMS 4 and 5 to reduce SAR. The pulse sequence includes both a fat saturation prepulse and slice select gradient reversal for fat suppression. Scan parameters were: TE/TR=110/3000 ms, 2.5×2.5×2.5mm3 resolution, 9 axial slices, 60/88 partial ky, and mixing time Tm = 31 ms. For each diffusion encoding pair: b=772 s/mm2, δ=17.6 Δ=19.8 ms, G = 50 mT/m, gradient rise time = 1 ms. A diffusion encoding scheme consisting of 12 B0 volumes, 30 diffusion-encoded volumes with the first diffusion-encoding gradient pair (G1) parallel to the second diffusion-encoding gradient pair (G2), 30 diffusion-encoded volumes with G1 antiparallel to G2, and 60 diffusion-encoded volumes with G1 perpendicular to G2 was acquired in a scan time of 7 min. Images were acquired with SMS factor 1-5. A FOV/3 shift was performed using blipped CAIPI9-10 for SMS 3-5, while a FOV/2 shift was employed for SMS 2. An additional 50 B0 images were acquired in order to compute the temporal SNR at each SMS factor.
Data Analysis: The diffusion images were reconstructed using split-slice grappa11 with SENSE1 coil combination12. The reconstructed images were corrected for EPI and eddy current distortions and bulk motion using “eddy” and from the FMRIB Software Library. Fractional eccentricity maps were computed from the diffusion images. The SNR of the B0 images was calculated by dividing the mean of the signal over the repetitions by the standard deviation. Image volumes displaying motion artifacts were excluded from the calculation. The SNR maps were smoothed with a Gaussian filter with a width of three voxels and a standard deviation of one voxel.
Results and Discussion
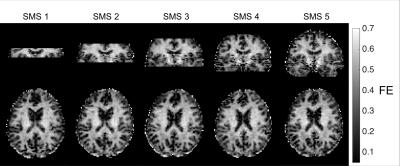
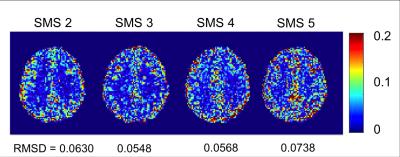
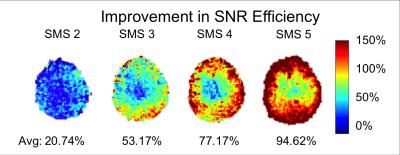
The FE maps computed from the diffusion data are shown in Figure 1. As seen in Figure 1, the SMS sequence is capable of extending the slice coverage of the DDE scan while maintaining a clinically feasible scan time. The FE maps from the SMS sequence exhibit no visible artifacts with only a slight reduction in SNR. As seen in Figure 2, the FE maps generated using the SMS and conventional DDE sequence are in close agreement with some variations in FE near tissue boundaries, which we attribute to small errors in image registration. The improvement in SNR efficiency of the SMS sequence was measured by scaling the ratio of the SNR of the SMS sequence to the conventional sequence by the square root of the SMS factor. The SMS sequence resulted in an average SNR efficiency improvement between 20 and 100 percent compared to the conventional sequence. The SNR efficiency increased approximately linearly from SMS factors 2 through 5. As expected, the improvement in SNR efficiency was higher at the periphery and lower at the center of the brain (Figure 4). Therefore, the SMS DDE sequence is particularly well-suited to interrogating the complicated tissue structures of the cerebral cortex.Conclusions
In this work, we present a double diffusion encoding pulse sequence with SMS capabilities. SMS allows DDE with full brain coverage (2.5mm isotropic resolution and 45 slices) in a clinically feasible scan time (7 min). SMS achieved up to 95% improvement in SNR efficiency and opens the possibility to other DDE acquisitions at higher resolution or with more complex double diffusion encoding schemes13 in a reasonable scan time. Acknowledgements
Funding provided by an NSF-GRFP, a Li-Ka Shing-Oxford-Stanford Big Data in Human Health Seed grant, NIH: R01-NS095985, S10-RR026351, P41-EB015891, Stanford Radiology Angel Funds, and GE Healthcare.References
1 Mitra, P. P. (1995). Multiple wave-vector extensions of the NMR pulsed-field-gradient spin-echo diffusion measurement. Phys Rev B Condens Matter, 51(21), 15074-15078.
2 Jespersen, S. N., Lundell, H., Sonderby, C. K., & Dyrby, T. B. (2013). Orientationally invariant metrics of apparent compartment eccentricity from double pulsed field gradient diffusion experiments. NMR Biomed, 26(12), 1647-1662. doi:10.1002/nbm.2999
3 Shemesh, N., & Cohen, Y. (2011). Microscopic and compartment shape anisotropies in gray and white matter revealed by angular bipolar double-PFG MR. Magn Reson Med, 65(5), 1216-1227. doi:10.1002/mrm.22738
4 Ozarslan, E., & Basser, P. J. (2008). Microscopic anisotropy revealed by NMR double pulsed field gradient experiments with arbitrary timing parameters. J Chem Phys, 128(15), 154511. doi:10.1063/1.2905765
5 Lawrenz, M., & Finsterbusch, J. (2011). Detection of microscopic diffusion anisotropy on a whole-body MR system with double wave vector imaging. Magn Reson Med, 66(5), 1405-1415. doi:10.1002/mrm.22934
6 Lawrenz, M., & Finsterbusch, J. (2015). Mapping measures of microscopic anisotropy in human brain white matter in vivo with double-wave-vector diffusion-weighted imaging. Magn Reson Med, 73(2), 773-783. doi:10.1002/mrm.25140
7 Setsompop, K., Cohen-Adad, J., Gagoski, B. A., Raij, T., Yendiki, A., Keil, B., . . . Wald, L. L. (2012). Improving diffusion MRI using simultaneous multi-slice echo planar imaging. Neuroimage, 63(1), 569-580. doi:10.1016/j.neuroimage.2012.06.033
8 Conolly S, Nishimura D, Macovski A, Glover G. Variable-rate selective excitation. J Magn Reson 1988;78:440–458.
9 Breuer, F. A., Blaimer, M., Heidemann, R. M., Mueller, M. F., Griswold, M. A., & Jakob, P. M. (2005). Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magnetic Resonance in Medicine, 53(3), 684-691. doi:10.1002/mrm.20401
10 Setsompop, K., Gagoski, B. A., Polimeni, J. R., Witzel, T., Wedeen, V. J., & Wald, L. L. (2012). Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med, 67(5), 1210-1224. doi:10.1002/mrm.23097
11 Cauley, S. F., Polimeni, J. R., Bhat, H., Wald, L. L., & Setsompop, K. (2014). Interslice leakage artifact reduction technique for simultaneous multislice acquisitions. Magn Reson Med, 72(1), 93-102. doi:10.1002/mrm.24898
12 Sotiropoulos, S. N., Moeller, S., Jbabdi, S., Xu, J., Andersson, J. L., Auerbach, E. J., … Lenglet, C. (2013). Effects of Image Reconstruction on Fibre Orientation Mapping from Multi-channel Diffusion MRI: Reducing the Noise Floor Using SENSE. Magnetic Resonance in Medicine?: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine, 70(6), 10.1002/mrm.24623. http://doi.org/10.1002/mrm.24623
13 Lasic S, Nilsson M, Latt J, Stahlberg F, Topgaard D. Apparent exchange rate mapping with diffusion MRI. Magn Reson Med 2011; 66:356–365.
Figures