3337
Rotating Single-shot Acquisition (RoSA) combined with parallel imaging for fast high-resolution diffusion imaging1Radiology and Imaging Science, Indiana University, Indianapolis, IN, United States, 2Siemens Medical Solutions, Inc. , Hanover, NH, United States, 3Siemens Medical Solutions, Inc. , Morrisville, NC, United States
Synopsis
High-resolution diffusion-weighted MRI often relies on multi-shot acquisitions, which suffer from long acquisition time. Rotating single-shot acquisition (RoSA) was proposed to accelerate high-resolution diffusion MRI by taking advantage of the similarity between diffusion-weighted images. In RoSA only a strip of k-space (i.e., blade) per diffusion direction is acquired, and high-resolution image was achieved via composite reconstruction. In this work, we implemented RoSA with parallel imaging. We demonstrated that improved data quality and imaging speed was achieved in both simulation and in human data.
Introduction
High-resolution diffusion imaging with an in-plane resolution of 1×1mm2 or higher often relies on multi-shot acquisition which suffers from prolonged acquisition time. Recently, a rotating single-shot acquisition (RoSA) technique1 has been proposed to accelerate diffusion imaging by acquiring only a stripe of k-space (i.e., blade) per diffusion direction and reconstructing a full k-space by utilizing neighborhood similarity of diffusion weighted images. RoSA has shown to achieve a ~10 times acceleration compared to the state-of-art multi-shot technique. In RoSA, as the rotating blade is using echo-planar imaging (EPI), off-resonance effects cause blurring artifacts in images. Herein, we describe an approach of combining GeneRalized Autocalibrating Partial Parallel Acquisition (GRAPPA)2 with RoSA to improve data quality and imaging speed. The improvements are demonstrated in both simulation and human data.Method
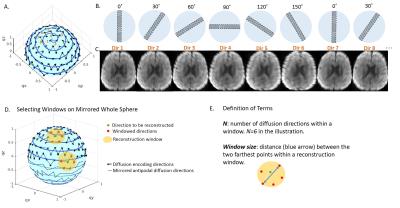
RoSA method: One single-shot EPI blade with short-axis in the readout direction was acquired per diffusion direction (Figure 1A,B). To optimize similarity of diffusion images within the reconstruction window (Figure 1D yellow circle), we minimized window size (defined in Figure 1E) by sorting all diffusion directions into a sequence that was closest to an Archimedean spherical spiral curve. The blade rotated as diffusion direction changed in this order (Figure 1B). A 30˚ rotation angle was used, therefore all blades were acquired at 6 unique angles (Figure 1A,B).
Upon reconstructing the i’th diffusion-weighted (DW) image (Figure 1D green dot), a subset of 6 DW blades with unique rotation angles whose directions were closest to the i’th direction on the sphere were used (Figure 1D red dots), forming a reconstruction window (Figure 1D yellow circle). These blades were combined in the k-space to form a composite image. The high-resolution DW image was then obtained by training the low-resolution DW image using the composite image, as described in ref. 1,3,4.
Combining with GRAPPA: With a reduction factor of R=3, all EPI blades were acquired by skipping every two phase-coding lines. A calibration dataset (36 lines) was acquired for each rotation angle using single-shot EPI, from where GRAPPA kernel was calculated and used for reconstructing undersampled blades of the corresponding rotation angle.
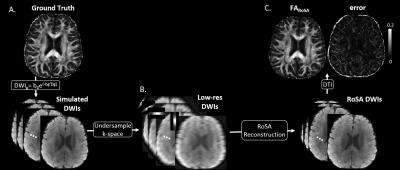
Simulation with tensor phantom: A 1mm isotropic brain DTI phantom was generated from a high quality DW multi-shot EPI sequence at b=1000s/mm2. The simulation of RoSA was shown in Figure 2. To simulate off-resonance effects with and without GRAPPA, a field map for the same subject was acquired and geometric distortion was formed in the image space using fugue (FSL).
Human data: RoSA was performed on a healthy volunteer at a 3.0T Siemens Prisma scanner with a 32-channel head coil with 1mm×1mm in-plane resolution, 60 diffusion directions and b-value = 1000s/mm2. A blade size of 48×256 and 30˚ rotation angle were chosen by taking into account tradeoffs between reconstruction window size (Figure 1E), off-resonance effects and k-space coverage. Other parameters included GRAPPA R=3, TE=59ms, NEX=2, 10 slices, acquisition time=2min. For comparison, conventional single-shot EPI (SS-EPI) and multi-shot EPI (RESOLVE) were acquired with matched resolution, diffusion directions, and slice thickness. The acquisition time was 2min and 24min respectively.
Results
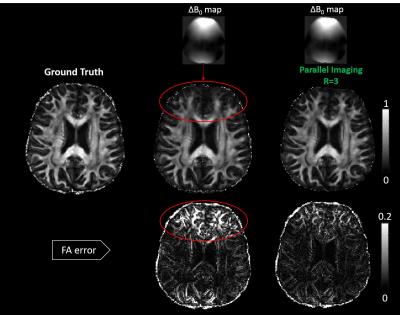
Figure 3 showed simulated RoSA FA (middle row) and FA errors (bottom) in the presence of off-resonance effects with and without parallel imaging. The red circles showed the blurring in the tip of the frontal lobes, which reduced significantly with parallel imaging (right column).
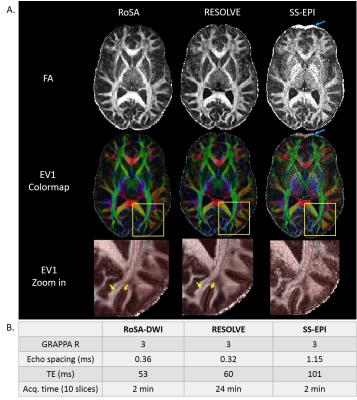
Figure 4 demonstrated FA, major eigenvector (EV1), and colormap for human data from different methods: RoSA, multi-shot EPI (RESOLVE), and conventional SS-EPI with matched resolution (1×1×2mm3). Critical acquisition parameters are listed in Figure 4B. With GRAPPA R=3, RoSA produced comparable image quality in computed DTI metrics to multi-shot EPI that was 12 times longer. Images of SS-EPI presented excessive noise due to long TE (101ms) and random variations in EV1 orientations. With an advantage of shorter TE (~53ms), RoSA and RESOLVE were able to show clear gyral stalks. In addition, images of RoSA and RESOLVE had reduced geometric distortions due to parallel imaging and very short echo spacing (~0.36ms). In contrast, despite the use of parallel imaging, the long echo spacing (1.15ms) in the SS-EPI sequence caused visible geometric distortions in the frontal lobes due to off-resonance effects of local field inhomogeneity (blue arrows).
Conclusion
RoSA offers high resolution and high image quality with more
than 10 times imaging efficiency. The imaging speed is achieved by utilizing
similarities in neighboring DW images, whereas the high image quality is
achieved by the nature of short-axis EPI blade and parallel imaging.
Acknowledgements
No acknowledgement found.References
1. Wen Q, Kodiweera C, Wu YC. “Windowed” Composite Reconstruction Improves Rotating Short-Axis High-Resolution DWI (RSA-DWI) in both Simulation and Human data. Proceedings of the 24th Annual Meeting ISMRM, Singapore, 2016; 516.
2. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002 Jun;47(6):1202-10.
3. Wu YC, Kodiweera C. Rotating short-axis EPI “Blades” as veering diffusion gradient directions with composite reconstruction (RSA). ISMRM 2014. P0666.
4. Mistretta CA, Wieben O, Velikina J, Block W, Perry J, Wu Y, Johnson K, Wu Y. Highly constrained backprojection for time-resolved MRI. Magn Reson Med. 2006. Jan;55(1):30-40.
Figures