3327
Evaluation of 129Xe-RBC signal dynamics and chemical shift in the cardiopulmonary circuit using hyperpolarized 129Xe NMR1Academic Unit of Radiology, University of Sheffield, Sheffield, United Kingdom
Synopsis
The signal dynamics of hyperpolarized 129Xe in the pulmonary capillaries and veins was evaluated by employing multi-TR pulse sequences, with TR values ranging from 100-6000ms. The ratio of signal from 129Xe dissolved in red blood cells (RBC) and tissue/plasma (TP) was found to increase for longer TRs whilst the 129Xe-RBC chemical shift was observed to decrease with increasing TR. This observed chemical shift difference is unprecedented, and understanding the nature of the underlying mechanisms causing this shift is crucial for future in vivo experiments using 129Xe-RBC chemical shift as a measure of blood oxygenation.
Purpose
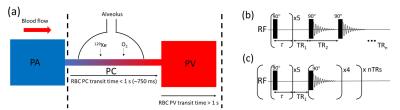
The sensitivity of the 129Xe chemical shift to red blood cell (RBC) oxygenation makes hyperpolarized 129Xe MR spectroscopy (MRS) a promising technique for measurement of pulmonary blood oxygenation in vivo. In previous studies of 129Xe-RBC chemical shift,1 the 129Xe signal was limited to the pulmonary capillaries (PCs) owing to the TR values (800ms) being of the order of the RBC capillary transit time (~750ms).2 In this study, TRs ranging from 100-6000ms were used in order to evaluate the 129Xe-RBC signal dynamics as blood travels through the capillaries downstream into the pulmonary veins (PVs) (Fig. 1a).Methods
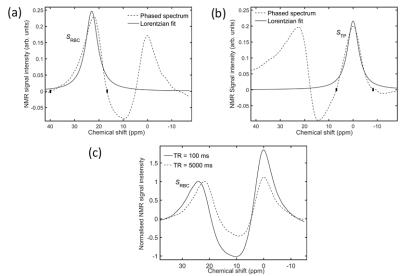
Hyperpolarized 129Xe pulmonary MRS was performed on three healthy male volunteers (aged 26, 30 and 33) on a clinical 3T (Philips, Achieva) scanner with a quadrature flex T-R coil. All scans were performed at breath-hold apnea after inhalation of 1L gas (500mL Xe, 500mL N2) from functional residual capacity. Two multi-TR sequences were implemented, each preceded by five 90° saturation pulses with TR = 100ms. The first sequence (Fig. 1b) was performed on all volunteers and comprised a single breath-hold with TR values: 100-1500ms with ΔTR = 100ms; and 2000-6000ms with ΔTR = 500ms, giving a total breath-hold time of ~1 min. The second sequence (Fig. 1c) was performed on the 30-year-old volunteer only, and comprised five separate breath-holds, each with a single TR value (100, 750, 1500, 3000 and 6000ms). Parameters for all scans were: hard pulses with FA = 90°, spectral points = 256, BW = 3kHz. The 129Xe signal in RBCs, SRBC, and tissue/plasma (TP), STP, was determined by performing zeroth-order phasing on the associated resonances (Fig. 2a,b), followed by numerical integration between the y-axis zero crossings on either side of the resonant peaks. It was not possible to obtain good Lorentzian fits to the individual peaks, as shown in Fig. 2. The 129Xe-RBC chemical shift was determined using 0ppm for 129Xe-TP as a reference.Results and Discussion
In all volunteers, the signal ratio, SRBC/STP, was observed to increase with increasing TR (Fig. 3). Since each 90° RF pulse saturates both the RBC and TP signals, longer TR values enable polarized 129Xe nuclei to travel further downstream of the capillary bed, essentially sampling a larger effective 129Xe-blood volume, increasing SRBC. The 129Xe dissolved in tissue acts a large “DC offset”, as once saturated with Xe from the alveoli (<100ms) its concentration is approximately constant. Therefore, we assume that the additional blood plasma volume that is sampled at longer TRs makes a lesser contribution to STP, such that longer TRs result in larger SRBC/STP. In parallel, 129Xe-RBC chemical shift, δRBC, was observed to decrease with increasing TR, (~3ppm difference between TR = 100ms and 6000ms) whilst the 129Xe-TP chemical shift remained constant for all TRs. Fig. 2c shows a comparison of δRBC at TR = 100ms and 5000ms. In previous work,1,3 the 129Xe-RBC chemical shift was observed to decrease with decreasing blood oxygenation, suggesting that for longer TRs, the blood oxygenation is apparently lower. During capillary transit, the RBC oxygenation will increase and should remain close to 100% oxygen saturation as the RBCs travel through the veins. The observation that RBC oxygenation apparently decreases as the RBCs travel from the PCs into the PVs is contrary to this. The multi-TR scan with multiple breath-holds (Figure 1) was performed to evaluate whether the decrease in oxygenation for long TR values was a result of decreased alveolar PAO2 during breath-hold apnea over the ~1 min single breath-hold scan. As shown in Fig. 3b, the same trend of decreased δRBC was observed for the multiple breath-hold scans. However, the measured 129Xe-RBC chemical shifts of <20ppm for TR = 6000ms are of the same order as than those measured in vitro for fully deoxygenated blood (20.5ppm),1 suggesting that the observed decrease in δRBC is unlikely to result from RBC oxygen desaturation. Instead, the decrease in δRBC may result from a difference in bulk magnetic susceptibility between the PC and PV environments and/or differences in geometry of PC and PV vessels and their orientation with respect to B0, analogous to a recent report of differences in gas-phase 129Xe chemical shift in the conducting airways and alveoli of mice.4Conclusion
In this study, a decrease in the 129Xe-RBC chemical shift was observed for increasing TR values probing blood RBCs downstream of the pulmonary capillaries. The observed chemical shift difference is unprecedented, and understanding the nature of the underlying mechanisms causing the shift is crucial for future in vivo experiments using 129Xe-RBC chemical shift as a measure of blood oxygenation.Acknowledgements
No acknowledgement found.References
1. Norquay, G., et al., 129Xe Chemical Shift in Human Blood and Pulmonary Blood Oxygenation Measurement in Humans using Hyperpolarized 129Xe NMR. Magnetic Resonance in Medicine, 2016. DOI: 10.1002/mrm.26225. [Epub ahead of print]
2. West, J., Respiratory Physiology: The Essentials, 8th Ed, 2008.
3. Wolber, J., et al., Hyperpolarized 129Xe as a Probe for Blood Oxygenation. Magn Reson Med. 43(4):p.491-496, 2000.
4. Virgincar, R.S., et al., Establishing an Accurate Gas Phase Reference Frequency to Quantify 129Xe Chemical Shifts In Vivo. Magnetic Resonance in Medicine, 2016. DOI: 10.1002/mrm.26229. [Epub ahead of print].
Figures