3321
Ultra-Short Echo Time MRI Quantification of Airspace Enlargement in Bronchopulmonary Dysplasia and Alpha-1 Antitrypsin Deficiency: Parenchyma Destruction, Air trapping or Both?1Robarts Research Institute, London, ON, Canada, 2Division of Respirology, The University of Western Ontario, London, ON, Canada
Synopsis
UTE MRI signal-intensity has not yet been evaluated in young patients with AATD and BPD, where there may be different mechanisms of parenchyma and airway destruction. There is the potential to demonstrate UTE MRI as a quantitative-measurement-tool for longitudinal and treatment-response evaluations in these vulnerable patients. We evaluated UTE MRI and CT using -950HU and -856HU radiodensity-thresholds, and a ‘sliding-threshold’ for the UTE image, identifying regions with low-signal-intensity for multiple threshold values. Regions of normalized UTE signal-intensity <29 suggest airspace enlargement, and demonstrate the potential utility of UTE MRI in quantifying this without ionizing-radiation in AATD and BPD subjects.
Purpose
The onset of chronic-obstructive-pulmonary-disease (COPD) in early adulthood due to abnormalities in the lung airways and parenchyma are typical findings in patients with alpha-one antitrypsin deficiency (AATD)1 and adult survivors of neonatal bronchopulmonary dysplasia (BPD).2 Thoracic computed-tomography (CT) may be used to quantitatively evaluate lung abnormalities using well established CT density-thresholds for emphysema3 (<-950 Hounsfield-units (HU)) and air-trapping4 (<-856HU). Ultra-short-echo-time (UTE) MRI signal-intensity was also recently shown to spatially and quantitatively correlate with CT density and lung volume measurements in COPD patients.5 However, UTE MRI signal-intensity measurements have not yet been evaluated in young patients with obstructive lung disease stemming from AATD and BPD, where there may be different mechanisms of parenchyma and airway destruction. By characterizing these relationships, there is the potential to demonstrate UTE MRI as a quantitative measurement tool for longitudinal and treatment response evaluations in these vulnerable patients. Thus, the objective of this work was to investigate UTE MRI signal-intensity measurements in young patients with BPD and AATD. We hypothesized that MRI signal-intensity thresholds could be determined in a small group of patients to provide a way to quantify airspace-enlargement (emphysema) or gas-trapping (due to airway-remodeling).Methods
Participants and Image Acquisition:
Subjects with a clinical diagnosis of AATD or BPD provided written informed consent to ethics-board approved protocols and were evaluated using MRI, thoracic CT, spirometry and plethysmography. Imaging was performed on a whole body 3T Discovery MR750 (General Electric Health Care [GEHC], Milwaukee, WI) with broadband imaging capabilities. UTE MRI was obtained using a 32-channel torso coil (GEHC) and 3D-cones UTE research sequence (GEHC). Eighteen slices were acquired in the coronal plane with the following parameters in breath-hold: acquisition-time=15s, TE/TR/flip-angle=0.03ms/3.5ms/5°, field-of-view=40×40cm, matrix=200×200, NEX=1, and slice-thickness=10mm. UTE MR images were acquired at functional-residual-capacity (FRC)+1L and to enable direct comparisons, thoracic CT was acquired at the same lung volume, as previously described.5
Image Analysis:
UTE signal-intensity was normalized to mean liver signal-intensity and images were non-rigidly co-registered6 with corresponding CT slices, which were segmented using VIDA Pulmonary Workstation (VIDA diagnostics, Coralville, IA), as previously described.5 A CT mask density-threshold of -950HU was used to generate a mask identifying regions suggestive of emphysema, and a CT mask density-threshold of -856HU was used to generate a mask identifying regions suggestive of gas-trapping. A ‘sliding-threshold’ was used to generate a mask of the UTE image, identifying regions with low signal intensity for multiple threshold values. Each of these UTE masks were compared to the CT masks using the Dice-similarity-coefficient (DSC) and an overlap coefficient (OC) shown below that describes the overlap between the two masks normalized by the area of the CT mask, the standard to which the UTE mask is being compared.
($$$\frac{|CTmask\bigcap\>UTEmask|}{CTmask}$$$)
For each subject, the UTE signal-intensity threshold corresponding to the mask with the highest DSC value was identified. Due to the small sample size, the leave-one-out method was implemented to test the result of the DSC optimization.
Results
In this proof-of-concept demonstration, we evaluated four participants, including three adults with AATD (60±7 years) and one adult survivor of BPD (27 years), with post-salbutamol FEV1/FVC of 41±13% and 39%, respectively. Figure 1 shows CT and UTE MRI for representative AATD and BPD participants, with ≤-950HU and ≤-856HU CT masks and MRI normalized-signal-intensity ≤29 mask. The normalized-signal-intensity threshold was validated using the leave-one-out method and achieved optimum agreement with the CT threshold. When comparing to the -950HU mask, the mean DSC was 0.24±0.14 and mean OC was 0.60±0.18. When comparing to the -856HU mask, the mean DSC was 0.62±0.13 and mean OC was 0.55±0.20.Discussion
Regions of normalized UTE signal-intensity in AATD and BPD are suggestive of airspace enlargement but it is difficult to ascertain if this is due to emphysema or gas-trapping. It should be noted that the lung volumes imaged with CT/MRI were not the standard volumes previously reported to evaluate gas-trapping4 and emphysema,3 specifically at full-expiration and full-inspiration, respectively. As a result, there was a slight underestimate of the extent of emphysema and overestimate of the extent of gas-trapping, thus altering the DSC, since CT/MR images were acquired at FRC+1L. Further studies using UTE MRI may allow a better understanding of the mechanisms of parenchyma and airway destruction in AATD and BPD.Conclusion
Regions of normalized UTE signal-intensity less than 29 are suggestive of airspace enlargement, and demonstrate the potential utility of UTE MRI in quantifying this without the use of ionizing radiation in subjects with AATD and BPD.Acknowledgements
We thank Trevor Szekeres, RTMR and Dave Reese, RTMR for MRI of research volunteers.References
1. Kohnlein, T. & Welte, T. Alpha-1 antitrypsin deficiency: pathogenesis, clinical presentation, diagnosis, and treatment. Am J Med 121, 3-9 (2008).
2. Boucherat, O., Morissette, M.C., Provencher, S., Bonnet, S. & Maltais, F. Bridging Lung Development with Chronic Obstructive Pulmonary Disease. Relevance of Developmental Pathways in Chronic Obstructive Pulmonary Disease Pathogenesis. Am J Respir Crit Care Med 193, 362-375 (2016).
3. Hayhurst, M.D., et al. Diagnosis of pulmonary emphysema by computerised tomography. Lancet 2, 320-322 (1984).
4. Zach, J.A., et al. Quantitative computed tomography of the lungs and airways in healthy nonsmoking adults. Invest Radiol 47, 596-602 (2012).
5. Ma, W., et al. Ultra-short echo-time pulmonary MRI: evaluation and reproducibility in COPD subjects with and without bronchiectasis. J Magn Reson Imaging 41, 1465-1474 (2015).
6. Heinrich, M.P., et al. MIND: modality independent neighbourhood descriptor for multi-modal deformable registration. Medical image analysis 16, 1423-1435 (2012).
Figures
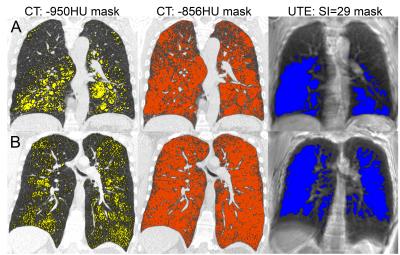
Figure 1. Thoracic CT and UTE MR images for representative AATD (top row) and BPD (bottom row) subjects
On the left, the yellow mask on the CT indicates areas of radiodensity <-950HU, suggestive of emphysema. In the center, the orange mask on the CT indicates areas of radiodensity <-856HU, suggestive of gas-trapping. On the right, the blue mask on the UTE MRI indicates areas of normalized SI below 29. A) The UTE mask clearly identifies areas of low radiodensity suggestive of emphysema. B) The UTE mask identifies areas of low radiodensity except near the diaphragm, where motion artifacts are present.