3320
Temporally-resolved volumetric imaging (4DMRI) of the lungs1Division of Radiological Physics; Department of Radiology, University of Basel Hospital, Basel, Switzerland, 2Department of Biomedical Engineering, University of Basel, Basel, Switzerland, 3Medical Image Analysis Center, University of Basel, Basel, Switzerland
Synopsis
Treatment planning relies on accurate organ motion modeling. Temporally-resolved volumetric imaging (4DMRI) of the lungs using a recently developed ultra fast steady state free precession sequence was attempted. No artifacts were present in the lungs, while image stacking was accurate with no significant image sorting issues.
Introduction
Treatment planning in interventional MRI and radiotherapy relies vitally on accurate organ motion modeling (1). To this end, different methods have been devised in the past to acquire temporally-resolved three-dimensional images (4DMRI) of the moving organs. One of the most sophisticated methods is based on the 2D multi-slice interleaved acquisitions of data and navigator slices in order to capture even subtle motion of organs during therapy, organ drifts (2). Balanced steady-state free precession (bSSFP) is a promising MR pulse sequence to image organ motion due to its temporal resolution and high signal-to-noise ratio, while in-flow effect increases the signal of blood vessels, which is desirable in motion tracking algorithms. However, imaging with bSSFP-type of sequences in the lungs is challenging due to banding artifacts, for which riddance short TRs are required. Recently, a pulse sequence for lung imaging, termed ultra-fast balanced steady state free precession (ufSSFP) was proposed (3), offering repetition times close to 1ms, thus artifact-free imaging at 1.5T. Here, the 4DMRI imaging method is adapted to offer ufSSFP repetition times from which lung motion models can be derived, to be used in radiotherapy or interventional MRI treatments of the lung.
Methods
A 4DMRI sequence was implement on a clinical 1.5T MR system (gradient field maximum amplitude of 40 mT/m and slew rate of 200 mT/(m × ms)). Excitation pulses, gradient switching patterns, and partial echoes were adjusted to reduce echo and repetition time as described in (3). The acquisition scheme was interleaved, i.e. data and navigator slices were acquired one after another as in (2). Data slices were positioned to cover the whole lungs, while the navigator was placed in the right part of the lungs to capture the main head-feet motion. Data and navigator slices were of sagittal orientation. MR signal was received using a body coil made of 6 channels, together with spine coils built-in in the patient table. The adjustment of shim option was set to a default or tune up. All data analysis and visualization were performed offline. The following imaging parameters were used: TE/TR = 0.88/1.5 ms, bandwidth = 1300 Hz/pixel, flip angle = 34°, number of slices = 52, parallel acceleration factor = 2, number of measurements = 50, total imaging time = 10 min, field of view = 288 × 420 mm2, in-plane resolution = 2.19 × 2.19 mm2, imaging matrix = 132 × 192, slice thickness = 8 mm, acquisition time per image = 124.5 ms resulting in a sampling frequency of 8.03 Hz. In vivo experiments were performed on three healthy volunteers.Results
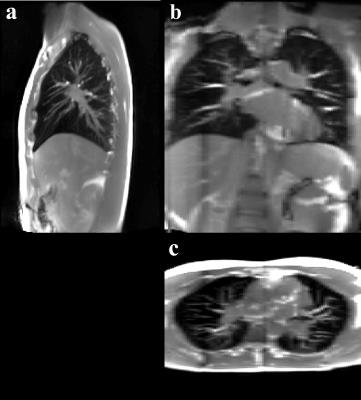
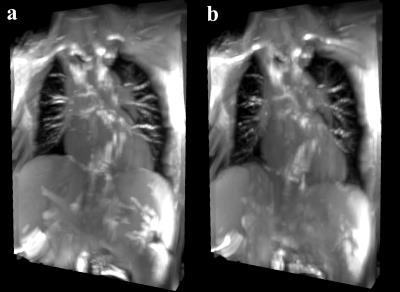
Exemplary images of data and navigator slices are shown in Fig. 1a and 1b, respectively. While the lungs are without any artifacts, there are still some remaining banding artifacts in the lower part of the liver and around the heart (blue arrows in a), caused by the magnetic field inhomogeneities. The horizontal red line in Fig. 1b, i.e. navigator, provides a visual clue of the organ motion amplitude. In Fig. 2, a 3D stack of images at the end of the expiration are shown in different orientations. Fig. 3a and 3b show 3D stacks at the end of the expiration and in the inspiration, respectively.Discussion
The ultra short echo and repetition time of the ufSSFP provide imaging free of banding artifacts in the lungs. The signal obtained from the lung tissue using this method had a high signal-to-noise ratio and contrast-to-noise ratio. Shortening of repetition time yielded the reduction of total imaging time per image, which translated into high sampling frequency of the breathing motion that could potentially increase accuracy of organ motion models. Images are accurately assigned to theirs corresponding respiratory phases using the 2D navigator, which could be asserted from viewing the 3D stacks from a coronal and axial view. Repeated acquisition of the navigator, which was kept at the same position caused signal saturation that is visible on the 3D stacks. While not of a significant issue, in order to avoid it, one could image the lungs segmented as to avoid overlapping of the navigator and data slices volume. External tracking methods could be attempted in future to provide additional or even complete tracking information, e.g. using a different imaging modality.Conclusion
4DMRI imaging method was successfully demonstrated in the lungs providing artifact-free images with high temporal resolution. Image stacking was accurate with no significant image sorting issues.Acknowledgements
We thank Swiss National Science Foundation (SNF) for financing this research. Grant number: 320030_163330References
1. Nguyen D et al. Computerized triplet beam orientation optimization for MRI-guided Co-60 radiotherapy. Med Phys. 2016;43:5667; 2. von Siebenthal M et al. 4D MR imaging of respiratory organ motion and its variability. Phys Med Biol. 2007;52:1547-1564; 3. Bieri O. Ultra-fast steady state free precession and its application to in vivo 1H morphological and functional lung imaging at 1.5 Tesla. Magn Reson Med. 2013;70:657-663.Figures