3319
Hyperpolarized Helium-3 MRI Insights into Subtypes of Emphysema in Chronic Obstructive Pulmonary Disease (COPD)1Department of Medicine, Columbia University Medical Center, New York, NY, United States, 2Taub Institute for Research on Alzheimer's Disease and the Aging Brain, Columbia University Medical Center, New York, NY, United States, 3Department of Radiology, Columbia University Medical Center, New York, NY, United States, 4Department of Infection, Immunity & Cardiovascular Disease, University of Sheffield, Sheffield, United Kingdom, 5Department of Radiology, Weill Cornell Medical Center, 6Department of Physics, Columbia University Medical Center, New York, NY, United States, 7Department of Radiology, University of Iowa Carver College of Medicine, IA, United States
Synopsis
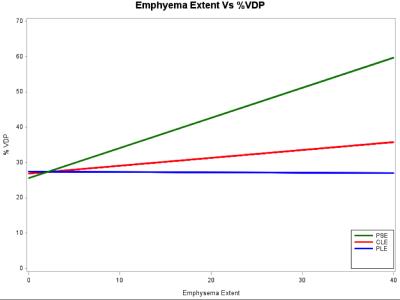
To examine the association of ventilation defects on 3He MRI with emphysema subtypes, participants (n=41) between 60 and 85 years of age with 10 or more packyears of smoking were studied. Ventilation defect percentage (VDP) was associated with percent emphysema (r =0.43; p=0.008) and, with borderline statistical significance, extent of visual emphysema (r=0.31; p=0.07). There was no relationship between VDP and extent of centrilobular (p=0.61) or panlobular (P=0.98) emphysema. VDP was associated with extent of paraseptal emphysema (PSE) (r=0.42; p=0.01). These findings suggest that small airways disease may be a component of PSE but not other subtypes of emphysema.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide. COPD is characterized by airflow limitation and the main causes are smoking, air pollution and biomass fuels. COPD overlaps with pulmonary emphysema, which is defined as enlargement of airspaces distal to the terminal bronchiole accompanied by destruction of their walls. Emphysema is a heterogeneous disease with three classical pathologic subtypes: centrilobular (CLE), panlobular (PLE), and paraseptal emphysema (PSE). These subtypes have different risk factors, histology, radiologic appearance, and distributions (e.g. CLE: upper zone; PLE: lower lung zone; PSE: lung periphery). The small airways disease (SAD) hypothesis suggests that SAD contributes to the development of airflow limitation and COPD and secondarily emphysema, whereas the vascular and other hypotheses of emphysema do not connect SAD to emphysema. SAD may contribute to specific emphysema subtypes, yet there is limited information on the associations of SAD and emphysema subtypes. Hyperpolarized (HP) noble gas magnetic resonance (MR), including helium-3 (3He), provides a non-invasive measure gas trapping of SAD in smokers with sub-clinical airflow limitation, in addition to reflecting bullous emphysema1. The purpose of this study is to examine the association of ventilation defects on 3He MRI with emphysema subtypes among older smokers with and without COPD.METHODS
The local Institutional Review Board approved this Health Insurance Portability and Accountability Act compliant study. Participants (n=41) were between 60 and 85 years of age with 10 or more packyears of smoking. 58% of the participants had COPD defined by standard spirometric criteria (28% mild, 30% moderate COPD and severe COPD). All participants underwent standardized full-lung CT scans following the SPIROMICS inspiratory protocol2. Percent emphysema was defined as the percentage of the lung below the attenuation threshold of -950 HU. CT scans were assessed for the classic emphysema subtypes following a standardized and reproducible protocol. Ventilation MR imaging was conducted using a Phillips 3T scanner with a flexible wrap-around 3He RF coil (CMRS). The 3He was polarized to 29+/-5.7% using a GE spin exchange polarizer. After a proton survey scan, coronal ventilation images covering the entire lung were acquired after the subject exhaled to residual volume and then inhaled 300 cm3 of HP 3He mixed with 700 cm3 N2. During the same breath hold the proton images with the slices identical to the 3He images were obtained. A semi-automated approach was used to analyze the 3He ventilation images to generate ventilation defect percentage (VDP). Regional ventilation defects were evaluated by three radiologists and the ventilation defect score (VDS) was generated for upper and lower regions of the lungs. Ventilation defects were identified as lung region of signal void excluding mediastinal and vascular areas. Pearson’s correlations between percent VDP and percent predicted FEV1, percent emphysema and extent of visual emphysema were calculated. Linear regression analyses adjusted for age, sex and smoking status, were used to determine association between percent VDP and outcomes. Statistical analyses were performed by using SAS.RESULTS
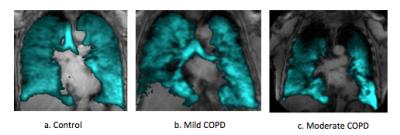
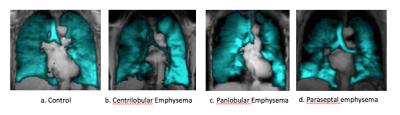
Among 41 the participants, higher VDP was associated with COPD (P=0.05) and inversely associated with F FEV1 percent predicted (r=-0.50; p=0.002; Figure 1). VDP was associated with percent emphysema (r =0.43; p=0.008) and, with borderline statistical significance, extent of visual emphysema (r=0.31; p=0.07). Among emphysema subgroups, there was no evidence for a relationship between VDP and extent of CLE (p=0.61) or PLE (P=0.98). In contrast, VDP was associated with extent of PSE (r=0.42; p=0.01). The linear relationships between VDP and emphysema subtypes is shown in Figure 2. There was no correlation between emphysema extent vs VDS region by region. Figure 3 and Figure 4 show center-section of coronal 3He ventilation images overlaid on proton lung images by COPD severity and for emphysema subtypes for patients with approximately similar severity of COPD. Ventilation defects were shown clearly as regions of signal void.DISCUSSION AND CONCLUSION
VDP, a measure of gas trapping and small airways disease, was associated with airflow limitation and with PSE but there was no evidence of association with CLE and PLE among older smokers, many with COPD. CLE is the classic smoking related emphysema but there was no-to-little gas trapping with CLE in this study. PLE is usually unrelated to smoking and there was no evidence for association with gas trapping. PSE is smoking-related, and gas trapping and small airway disease may have contributed to PSE. These preliminary findings suggest that small airways disease may be a component of PSE but not other subtypes of emphysema.FUNDING
NIH/NHLBI R01-HL093081, R01-HL077612, R01-HL075476Acknowledgements
No acknowledgement found.References
1. Dante P. I. Capaldi · Nanxi Zha · Fumin Guo, et al. Pulmonary imaging Biomarkers of gas Trapping and emphysema in COPD: 3He MR Imaging and CT Parametric Response Maps, Radiology. 2016 ;279(2):597-608
2. Sieren JP, Newell Jr JD, Barr RG, et al. SPIROMICS protocol for multicenter quantitative CT to phenotype the lungs, Am J Respir Crit Care Med. 2016 ;194(7):794-80
3. Smith BM, Austin JH, Newell JD Jr, et al. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med. 2014 Jan;127(1):94.e7-23.
Figures