3311
Repeatability of regional lung ventilation quantification using 19F fluorinated gas washout magnetic resonance imaging in free breathing1Institute of Radiology, Hannover Medical School, Hannover, Germany, 2Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Hannover, Germany, 3Clinical Airway Research, Fraunhofer Institute for Toxicology and Experimental Medicine, Hannover, Germany
Synopsis
Since quantification of regional lung ventilation using 19F fluorinated gas washout imaging in free breathing is feasible even in obstructed lungs, it may improve diagnosis, monitoring and therapy of obstructive lung diseases like asthma and COPD. However, for application in clinical studies the knowledge of the accuracy of this technique is important. Repeatability of the 19F gas washout parameters washout time, number of breaths and fractional ventilation between to scans was assessed in eight healthy volunteers. Due to the excellent repeatability of the number of breaths and fractional ventilation, regional lung ventilation can be accurately quantified using 19F gas washout MRI in free breathing.
Introduction
Direct visualization of regional lung ventilation using either hyperpolarized gases (3He, 129Xe) or thermally polarized fluorinated (19F) gases (SF6, C2F6, C3F8 etc.) may improve diagnosis, monitoring and therapy of obstructive lung diseases like asthma and chronic obstructive lung disease (COPD). Hyperpolarized gas magnetic resonance imaging (MRI) is typically applied by static imaging after a single inhalation of approximately 1 L of the trace gas. However, regional lung ventilation quantification may be challenging in regions of delayed gas uptake1. Quantification of gas washin / washout time in multi-breath hyperpolarized gas MRI is very complex as it requires correction of magnetization depletion due to oxygen-dependent T1 relaxation and application of radio frequency pulses2,3. Alternatively, multi-breath imaging with normoxic fluorinated gases is technically much simpler, since attenuation of 19F MR signal by oxygen can be neglected 4-8. Despite the significantly lower signal-to-noise ratio of 19F gas MRI compared to hyperpolarized gas MRI, regional quantification of lung ventilation using 19F gas washout MRI is feasible in free breathing even in COPD patients5. However, for application in longitudinal patient studies, knowledge of its repeatability is of high interest. The purpose of this study was to investigate the repeatability between two scans of regional lung ventilation quantification using 19F gas washout MRI in free breathing in healthy volunteers.Methods
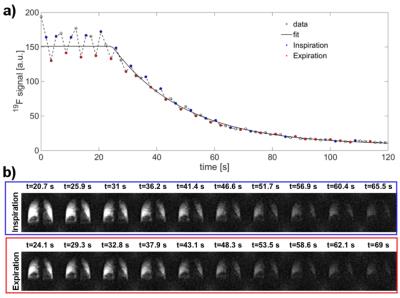
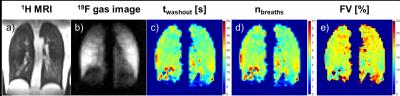
The study was approved by the institutional ethical review board and all subjects gave written informed consent. Eight healthy non-smokers (age: 18-69 years, 3 females) were examined twice within one week with both fluorinated gas MRI and lung function test. MRI was performed on a 1.5T scanner (Siemens Avanto) using a dedicated 19F coil (birdcage transmit coil/ 16 channel phased-array receive coil). After inhalation of 30 L of a normoxic gas mixture of perfluoropropane (C3F8) (21% O2 / 79% C3F8) via a close fitting face mask and then switching to pure oxygen, 19F gas washout MRI was performed in free breathing using a 3D spoiled gradient echo sequence (TE: 5.1ms, TR: 15 ms, FOV: 500 x 500 x 300 mm3 , matrix size: 64 x 64 x 6, GRAPPA factor: 2, bandwidth: 140 Hz/ pixel, 70 3D data-sets with a temporal resolution of 1.7 s covering the whole lung). For safety reasons, over the whole scan time of 19F MRI, physiologic parameters of the examined subjects were supervised by a physician who could switch to pure oxygen in case of hypoxia or discomfort9. After automatic selection of the expiratory time frames, regional 19F gas washout time (twashout) was determined by mono-exponential fitting (Figure 1). In addition, regional 19F gas washout was quantified in number of breaths (nbreaths) and by fractional ventilation (FV). After manual segmentation of the lung, global lung ventilation was determined by calculating median of twashout, nbreaths and FV. Repeatability of the repeated scans was analyzed with Bland-Altman plot analysis and by calculating coefficient of variation (COV) among all subjects.Results
Exemplary maps of twashout, nbreaths and FV derived by 19F gas washout MRI in free breathing can be found in Figure 2. Bland-Altman plots of median twashout, nbreaths and FV over the whole lung compared within the same subject for two different scan times are shown in Figure 3. Lowest COV was found for FV (4.4%), followed by nbreaths (8.7%) and twashout (24.3%).Discussion
While a good repeatability was found for nbreaths and FV, twashout showed a high variability. 19F washout measurements were performed in free breathing. Therefore, variations of respiration regarding to tidal volume and respiratory frequency are possible sources of error. The washout time of fluorinated gas MRI decreases with both increased tidal volume and increased respiratory frequency. This explains the notably higher variability of twashout compared to nbreaths and FV which are at least compensated for the respiratory frequency but not for tidal volume. The mean respiratory frequency was determined by analysis of the gas washout curve. However, due to the temporal resolution of 1.7 s, accurate estimation of the respiratory frequency is limited to less than 18 breaths per minute. In one subject, for scan I, a respiratory frequency near the maximum limit was found. Correspondingly, this subject showed the lowest repeatability for nbreaths (difference between scan I and scan II: 1.9 breaths) and FV (difference between scan I and scan II: 1.9 breaths: 2.35%). Potentially, a spirometer could be used to measure and adjust for variation of tidal volume, which may even further increase repeatability of the washout measurement.Conclusion
The excellent repeatability found for nbreaths and FV allows accurate quantification of regional lung ventilation using 19F gas washout MRI in free breathing.
Acknowledgements
This work was supported by the German Centre for Lung Research (DZL), Regenerative Biology and Reconstructive Therapies (REBIRTH) and Fritz-Behrens-Stiftung, Hannover.References
1. Marshall H, Deppe MH, Parra-Robles J, et al. Direct visualisation of collateral ventilation in COPD with hyperpolarised gas MRI. Thorax 2012;67(7):613–617.
2. Horn FC, Deppe MH, Marshall H, et al. Quantification of regional fractional ventilation in human subjects by measurement of hyperpolarized 3He washout with 2D and 3D MRI. J Appl Physiol 2014;116(2):129–139.
3. Hamedani H, Clapp JT, Kadlecek SJ, et al. Regional Fractional Ventilation by Using Multibreath Wash-in (3)He MR Imaging. Radiology 2016;279(3):917-2.
4. Halaweish AF, Moon RE, Foster WM et al. Perfluoropropane gas as a magnetic resonance lung imaging contrast agent in humans. Chest 2013;144,(4):1300–1310.
5. Gutberlet M, Kaireit T, Voskrebenzev A, et al. Real-Time Dynamic Fluorinated Gas MRI in Free Breathing for Mapping of Regional Lung Ventilation in Patients with COPD and Healthy Volunteers Using a 16 Channel Receive Coil at 1.5T. Proc. Intl. Soc. Mag. Reson. Med. 2016;25:1140.
6. Ouriadov AV, Fox MS, Couch MJ, et al. In vivo regional ventilation mapping using fluorinated gas MRI with an x-centric FGRE method. Magn. Reson. Med. 2015;74(2):550–557.
7. Wolf U, Scholz A, Heussel CP, et al. Subsecond fluorine-19 MRI of the lung. Magn. Reson. Med. 2006;55(4):948–951.
8. Couch MJ, Fox SC, Viel C, et al. Fractional ventilation mapping using inert fluorinated gas MRI in rat models of inflammation and fibrosis. NMR Biomed. 2016;29(5):545–552.
9. Halaweish AF, Charles HC. Physiorack: An integrated MRI safe/conditional, Gas delivery, respiratory gating, and subject monitoring solution for structural and functional assessments of pulmonary function. J. Magn. Reson. Imaging 2014;39(3):735–741, 2014.
Figures