3309
A preliminary study of in-vivo visualization of oxide oxide enhancement in focal inflammatory lesion of lung parenchyma using a 3D radial gradient-echo-based UTE sequence (CODE)Soon Ho Yoon1, Suh-Young Lee2, Chanhee Lee3, Jinil Park3,4, Jin Mo Goo1, and Jang-Yeon Park3,4
1Department of radiology, Seoul National University Hospital, Seoul University College of Medicine, Seoul, Korea, Republic of, 2Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea, Republic of, 3Center for Neuroscience Imaging Research, Institute for Basic Science (IBS), Suwon-si, Gyeonggi-do, Korea, Republic of, 4Department of Biomedical Engineering, Sungkyunkwan University, Suwon-si, Gyeonggi-do, Korea, Republic of
Synopsis
Dual-echo ultrashort echo-time CODE pulmonary MRI preliminarily succeeded in generating a positive contrast of iron oxide in rabbits with granulomatous lung disease, by using clinically-usable superparamagnetic iron-oxide nanoparticles, ferumoxytol, which was hardly achievable via conventional T2* MR sequence. This new pulmonary MR imaging biomarker possibly gives a chance to differentiate a benign inflammatory lesion from malignancy.
Purpose
T2* MR sequences are mainly used for visualizing macrophage uptake of the iron-oxide agents, but they are vulnerable to magnetic field inhomogeneity and cardiopulmonary motion in the lung parenchyma and mediastinum (1). Accordingly, the observation of iron-oxide contrast is fundamentally limited in the thorax when using T2* MR sequences. Detection of iron-oxide contrast agents in the thorax by dual-echo acquisition and subtraction is a unique imaging biomarker only achievable in ultrashot echo-time (UTE) MRI (2). CODE (Concurrent Dephasing and Excitation) imaging, which was recently developed, is a 3D radial gradient-echo-based UTE sequence and avoids most of the challenges of conventional UTE sequences, acquiring an asymmetric gradient echo and still offering TE ≥ ~0.14ms on a clinical scanner (3). The purpose of our study was to preliminarily evaluate the technical feasibility of dual-echo CODE subtraction imaging for visualization of the iron-oxide enhancement in the focal lesion of lung parenchyma.Methods
This experiment was approved by our Institutional Animal Care and Use Committee (IACUC No. 13-0006-C2A0). Two New Zealand white rabbits weighing 3.0 - 3.5 kg was used for concurrent non-caseating granulomatous lesions and lung cancer. To establish concurrent lung cancer and non-caseating granulomatosis harboring pulmonary macrophages in the lung parenchyma, an uncuffed polyvinylchloride tube with an internal diameter of 2.5 mm and an outer diameter of 3.5 mm was used for tracheal intubation. Subsequently, a terumo guide wire and a 4Fr. Davis catheter were applied through the endotracheal tube on conventional fluoroscopy for the selection of lower lobes. A 1 ml of complete freund’s adjuvant was injected in the right lower lobe and a 1 ml of tumor suspension of VX2 carcinoma was inoculated in the left lower lobe. Dual-echo CODE imaging was performed in two rabbits three weeks after induction of concurrent granulomatous lesion and cancer (Table 1). Baseline dual-echo CODE images were acquired prior to the intravenous administration of ferumoxytol (Feraheme® , AMAG Pharmaceuticals, Lexington, MA, USA; 12 mg iron per kilogram body weight) into the marginal ear vein. Post-contrast dual-echo CODE images were acquired 24 hours after injection of iron-oxide nanoparticles. Before and after contrast injection, 1st-echo images were subtracted by 2nd-echo images to clarify the positive iron-oxide enhancement. After MR imaging, the rabbits were sacrificed with a lethal dose (90 mg/kg) of intravenously administered sodium pentobarbital (Pentothal; Choong Wae Pharmacy, Seoul, Korea). The bilateral lungs were isolated and fixed with 10% formalin. The lung tissues were embedded in paraffin, and the prepared consecutive sections (approximately 4-um thick) in the axial plane with a 4-um interval were stained with Prussian blue for identification of the iron-oxide nanoparticles. The immunohistochemistry staining was performed by applying mouse monoclonal anti-rabbit macrophage IgG1 (RAM 11, Dako) at a dilution of 1:500 in the section adjacent to a slide stained with Prussian blue to evaluate the intracellular uptake of iron-oxide nanoparticles by macrophage. To evaluate positive iron-oxide enhancement, a square ROI was differently applied three times for granulomatous lung lesion and lung cancer, respectively, at the subtracted axial images for calculating the signal-intensity (SI) ratio of these lesions (4). The mean SI ratio between the pre- and post-contrast subtracted images was calculated using the following formula: SI ratio = (SI lung lesion[post-USPIO] / SI muscle[post-USPIO]) / (SI lung lesion[pre-USPIO] / SI muscle[pre-USPIO]).Results
In two rabbits, the positive iron-oxide enhancement was depicted exclusively in the granulomatous lesions with the subtracted dual-echo CODE sequence (mean SI ratio, 2.44-2.98 for granulomatous lesion; 0.89-1.13 for lung cancer)(Figure 1). Prussian blue staining confirmed the presence of iron-oxide nanoparticles in the granulomatous lung lesion, but not in lung cancer on the histopathologic specimen. Corresponding anti-rabbit macrophage IgG–staining suggested an intracellular uptake of iron-oxide nanoparticles in macrophages.Discussion and conclusion
Our preliminary study succeeded in in-vivo visualization of positive enhancement of clinically applicable iron-oxide nanoparticles, ferumoxytol, exclusively in the granulomatous lung disease, not in the tumor.Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education(2010-0025744, 2013R1A1A2063746 and IBS-R015-D1).
References
(1) Bergin CB, Pauly JM, Macovski A. Lung Parenchyma: Projection Reconstruction MR Imaging. Radiology 1991;179:777-781. (2) Girard OM, Du J, Agemy L, et al. Optimization of iron oxide nanoparticle detection using ultrashort echo time pulse sequences: comparison of T1, T2*, and synergistic T1- T2* contrast mechanisms. Magnetic resonance in medicine. 2011;65(6):1649-60. (3) Park JY, Moeller S, Goerke U, et al. Short Echo-Time 3D Radial Gradient-Echo MRI Using Concurrent Dephasing and Excitation. Mag Reson Med. 2012;67:428-436. (4) Yoo RE, Choi SH, Cho HR, et al. Magnetic resonance imaging diagnosis of metastatic lymph nodes in a rabbit model: efficacy of PJY10, a new ultrasmall superparamagnetic iron oxide agent, with monodisperse iron oxide core and multiple-interaction ligands. PloS one. 2014;9(9):e107583.Figures

Table 1. MR acquisition parameters for in-vivo imaging of iron oxide enhancement
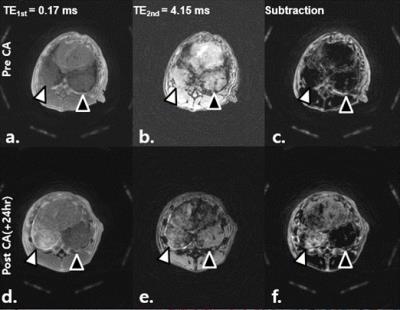
Figure 1.(A). Representative axial dual-echo CODE images (a-f) of concurrent granulomatous lung lesion (right lower lobe, white arrowhead) and lung cancer (left lower lobe; black arrowhead) in rabbits. Images were obtained before (a-c), and 24 hours after injection of ferumoxytol (d-f). Baseline 1st- and 2nd-echo images show similar mass-like consolidation (a, b). When subtracted, there was an identical signal nulling in those lesions (c). Ferumoxytol-enhanced MR images depict higher SI at the 1st-echo image and lower SI at 2nd-echo image in granulomatous lesion when compared to cancer (d-e). When subtracted, there is an obvious enhancement exclusively in granulomatous lesion (f).
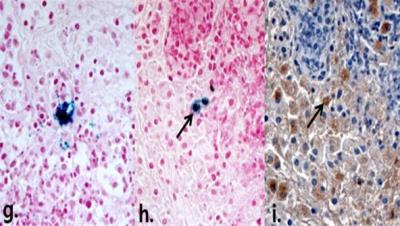
Figure 1.(B). Representative histopathologic Prussian blue, anti-rabbit macrophage IgG-stained images of granulomatous lung lesion. Prussian blue–stained slice shows dense intracellular accumulation of iron oxide nanoparticles (g). Prussian blue and corresponding anti-rabbit macrophage IgG–staining slices reveal intracellular uptake of the iron oxide nanoparticles in macrophages (h, i).