3305
In-Vivo semi-LASER Renal Magnetic Resonance Spectroscopy (MRS): Pilot Study in Healthy Volunteers1UHN, University of Toronto, Toronto, ON, Canada, 2Siemens Healthcare Limited
Synopsis
MR Spectroscopy (MRS) can provide chemical composition of in vivo tissue non-invasively. Detection of signature chemical composition in tumors which are expected based on knowledge of histopathology could enable discrimination between tumor subtypes and grade by MRS. In this feasibility study we demonstrate ability to perform with success renal MRS in health volunteers mapping normal signature composition of renal tissue and provide a platform to expand the work to renal cancer tumor composition mapping and discrimination therewith based on metabolite content.
Background
Renal Cell Carcinoma (RCC) accounts for over 90% of all kidney cancers with histological subtypes differing in morphology, genetics, tumor grade and prognosis. We propose to develop in vivo Magnetic Resonance Spectroscopy (MRS) for non-invasive detection of signature biochemical components in kidney cancer such as fat and choline for differentiation of RCC histologic subtypes (clear cell versus non clear cell RCC) and tumor grade.
Purpose
1) To test the feasibility of semi-LASER renal MRS in healthy volunteers and 2) establish signature chemical composition of normal renal tissue in order to develop it as a future tool for non-invasive histologic subtype differentiation of renal cancer via lipid and choline concentrations.Methods
14 healthy volunteers (7 male, 7 female, mean age 24 years) with no history of renal disease were recruited locally to participate in this pilot study. Informed consent was obtained from each subject in accordance with the Institutional Research Ethics Board All imaging exams were performed on a clinical 3T MRI system (MAGNETOM Verio; Siemens, Erlangen, Germany), using a body transmit RF coil and receive-only body matrix coil and spine matrix coil. Subjects were positioned head-first in a supine position with the kidneys centered near the isocentre. All imaging and spectroscopy acquisitions were performed with breath-hold at end-expiration. T2-weighted localizer image sets were first acquired in the coronal and axial planes to ensure optimal subject positioning (HASTE, 9 sec). This was followed by 2D anatomical image sets covering one kidney acquired in coronal, axial and sagittal planes (TrueFISP, 1.7x1.4 mm, slice 4.0 mm, TE/TR = 1.5/3.4 ms, 22 sec), which were used for subsequent positioning of the MRS voxel. The MRS voxel was manually positioned using the anatomical localizer image sets in all three planes. Care was taken to ensure the voxel was positioned entirely within the renal cortex of each patient, avoiding extra-renal tissue. Automated first- and second-order shimming was performed using a breath-hold 3D dual-echo gradient echo technique provided as a Siemens prototype for patient specific, volume selective shimming. Single voxel 1H spectra were acquired using a semi-LASER sequence (1), employing outer-volume suppression and VAPOR water suppression (10x10x10 mm voxel, TE/TR = 28/2000 ms, spectral bandwidth 2000 Hz, 2048 points). The transmitter frequency for all MRS acquisitions was set at 3.0 ppm, approximately half-way between the water and fat resonances at 4.7 ppm and 1.3 ppm, respectively; this was done to reach a compromise in chemical-shift induced displacement error between water and lipid resonances. Spectra were first acquired without water suppression with 8 averages in a single breath-hold (18 sec, including one dummy cycle). Water-suppressed spectra were then acquired from the same voxel in 8 separate breath-holds, with 8 averages each, for a total of 64 averages. Each individual MRS acquisition was saved separately to permit phase and frequency correction prior to signal summation. A short break of approximately 2 minutes was given between breath-holds to allow for subject recovery. Each transient MRS acquisition was exported for off-line processing using jMRUI v4.0 (2). All transients were automatically corrected for zero-order phase and frequency using the water resonance, or residual water resonance for water-suppressed acquisitions. Each corrected transient was manually inspected and phase and/or frequency correction was manually adjusted if necessary prior to the summation of the signals.Results
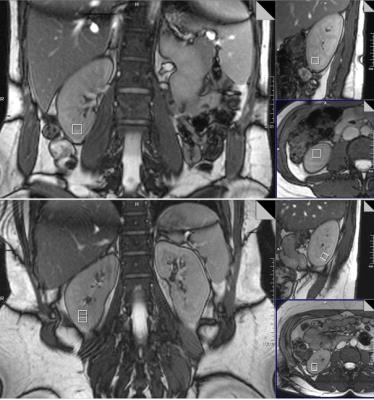
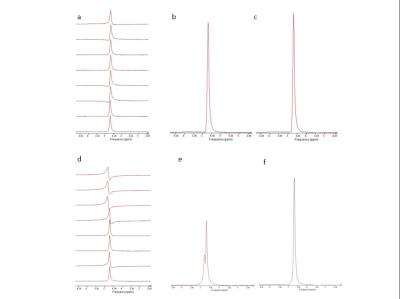
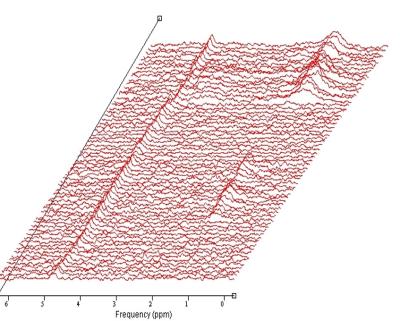
Of the 14 health volunteers,3 had difficulty holding their breath or remaining stationary,the remaining 11 were successfully examined. Given the small MRS voxel size used , 10x10x10 mm, it was always possible to position the voxel such that it was entirely within the renal cortex, usually at least 1-2 mm from the border of the kidney(Fig.1) . Most data sets exhibited relatively consistent phase and frequency from shot to shot in a given breath-hold(Fig.2), as observed in an earlier study by Katz-Brull et al. (3). However, data from some volunteers did show sufficient variation to make phase and frequency correction necessary to obtain optimal data quality prior to signal summation. No lipid resonance was observed in any spectra from the unsuppressed water acquisitions, either in individual transients or in corrected summed spectra. In the water-suppressed spectra, two of the 11 data sets did exhibit detectable levels of lipid resonance at 1.3 ppm; however, in both cases, the observed lipid signal was only present in less than half of the individual breath-holds, suggesting voxel contamination with extra-renal lipid due to patient motion(Fig.3). No signal from other metabolites, such as choline-containing compounds, was observed in any datasetDiscussion
No lipid or choline resonance was detected from the renal cortex of the normal volunteers opposed to previous literature (3,4) which had likely resulted from perinephric fat contamination from free breathing technique opposed to breathe hold semi-LASER MRS technique in our study. Thus detection of lipid in RCC which is expected in clear cell RCC may enable distinction from non-clear cell RCC by MRS .Further choline was not observed in normal renal cortex and identification of same may enable estimation of tumor grade.Conclusion
In-vivo semi-LASER Renal MRS is technically feasible and reproducible. Since normal renal tissue does not demonstrate detectable levels of lipid and choline,Semi-Laser MRS should be investigated as a non-invasive tool for RCC histological subtyping.Acknowledgements
1. Center for Magnetic Resonance Research(CMRR), Department of Radiology,University of Minnesota for semi-LASER sequence: Gulin Oz,Edward J. Auerbach ,Malgorzata Marjanska,Dingxin Wang
2. GRE Shim WIP author
References
1. Oez, G, Tkac I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: validation in the cerebellum and brainstem. MRM 2011; 65:901-910.
2. Naressi, A, Couturier, C, Devos, JM, Janssen, M, Mangeat, C, de Beer, R, Graveron-Demilly, D. Java-based graphical user interface for the MRUI quantitation package. MAGMA 2001; 12:141-52.
3. Katz-Brull R, Rofsky NM, Lenkinski RE. Breathhold abdominal and thoracic proton MR spectroscopy at 3T. MRM 2003; 50:461-467.
4. Rachel Katz-Brull,Neil M. Rofsky,Martina M. Morrin,Ivan Pedrosa,Daniel J. George,M. Dror Michaelson,Robert P. Marquis,Michal Maril,Carolina Noguera and Robert E. Lenkinski. Decreases in free cholesterol and fatty acid unsaturation in renal cell carcinoma demonstrated by breath-hold magnetic resonance spectroscopyAm J Physiol Renal Physiol 288: F637–F641, 2005.
Figures