3298
The Cortico-Medullary ADC Difference reduces inter-system variability in Renal Diffusion-Weighted Imaging1Radiology, University of Geneva, Geneva, Switzerland, 2Geneva University Hospitals
Synopsis
Our goal was to determine if the cortico-medullary Apparent Diffusion Coefficient difference (ΔADC), that previously exhibited a strong correlation with renal fibrosis, is independent of MR system. Comparison of ADC (cortex, medulla and Δ) over Siemens (1.5T AERA, 3T PRISMA, 3T SKYRA) and Philips (1.5T INGENIA, 3T PET-MR) systems was carried out in eight volunteers. Significant ADC differences were measured for the cortex and medulla independently using PRISMA and AERA and, for cortex of AERA and INGENIA (p<0.05). ΔADC corrected inter-scanner variability with no significant differences across all MR systems (p>0.05).
Purpose
Diffusion-Weighted (DW) Magnetic Resonance Imaging (MRI) in the kidney suffers from high variability across studies in the reported apparent diffusion coefficient (ADC) values (1). This variability limits use in clinical practice and makes the comparison of ADC data from different studies difficult despite attempts for a standardized protocol for DW-MRI inter-comparison (2). A key part of the problem of DW bias and variability comes from the use of MR systems with differences in hardware (scanner performance and gradients) (3), acquisition protocols and data post processing strategies and subject positioning within magnet (4), as well as physiological differences such as flow and tissue hydration level (5).The cortico-medullary difference, ΔADC is strongly correlated to renal interstitial fibrosis. It was already showed that ΔADC reduced physiological inter-subject variability by comparison to cortical or medullary ADC (6). However, an important remaining question is the variability of ΔADC between MR systems. We attempt in this study to determine if the cortico-medullary difference ΔADC could be a more robust parameter than cortical or medullary ADC for renal fibrosis assessment over different Siemens (SKYRA 3T, PRISMA 3T, AERA 1.5T) and Philips (PET-MR 3T, INGENIA 1.5T) MR systems.Methods
Subjects: Eight healthy volunteers, comprising 4 females and 4 males, with a mean age of 35 ± 9 years (range, 24-58 years), were recruited after informed consent. Subjects were scanned on all machines in the same session with no specific instructions for hydration state.
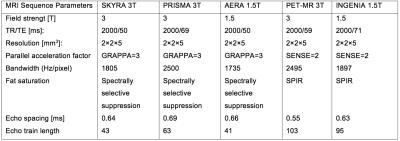
MRI: DW-MRI data were acquired on 5 different MR systems (1.5T and 3T, Siemens and Philips). All DW sequences were performed in breathhold with acquisition time between 18 and 26 seconds. The diffusion-encoding gradients were applied in 3 orthogonal directions with 3 b-values (0, 500, 700 s/mm2) and a bipolar diffusion scheme. Sequence DW parameters are summarized in table 1.
Image analysis: Apparent Diffusion Coefficient (ADC) from a monoexponential fit, was measured on quantitative ADC maps generated by the OsiriX ADC tool plugin (OsiriX Open source http://www.osirix-viewer.com). The b0 image was also used as a reference anatomical image for region of interest (ROI) positioning. ROIs were placed for analysis of both the cortex and medulla and ΔADC was defined as ΔADC = <ADC cortex> - <ADC medulla>
Statistical method: Comparison of mean ADC values (cortex, medulla and Δ) from the different MR systems was carried out using paired T test with p < 0.05 taken as statistically significant.
Results
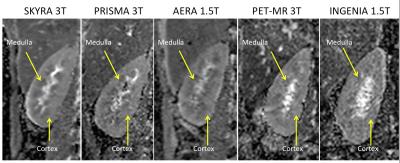
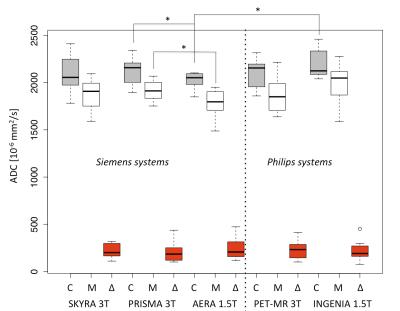
All DW images and ADC maps were suitable for ADC analysis with a visible cortico-medullary ADC difference as shown in Figure 1. Figure 2 shows a boxplot with mean ADC measured in the 8 healthy subjects. The paired T test showed no significant difference between the three 3T MR scanners for the cortical, medullary and ΔADC. However, significant differences were measured between Siemens MR scanners at 1.5 and 3T (p=0.02 for cortex and p=0.002 for medullae of PRISMA 3T and AERA 1.5T) and between scanners at 1.5T (p=0.04 for cortex of AERA and INGENIA). However, no significant differences was measured using the ΔADC parameter across all MR systems with all p>0.05.Discussion
Our goal was to verify if kidney ADC can be compared across multiple MR scanners and to reduce potential variability and bias, using the cortico-medullary difference. We found significant inter-vendor differences as well as significant difference between 1.5T and 3T with Siemens scanners by measuring ADC in the cortex and medulla independently. Inter-scanners variations were corrected in subjects through the use of the cortico-medullary difference ΔADC.Conclusion
Variability of ADC between different MR systems observed in the cortex and medulla is corrected by the use of the ΔADC. This makes ΔADC a strong biomarker of renal interstitial fibrosis.Acknowledgements
No acknowledgement found.References
1. Zhang JL, Sigmund EE, Chandarana H, et al. Variability of renal apparent diffusion coefficients: limitations of the monoexponential model for diffusion quantification. Radiology 2010;254(3):783-792.
2. Belli G, Busoni S, Ciccarone A, et al. Quality assurance multicenter comparison of different MR scanners for quantitative diffusion-weighted imaging. J Magn Reson Imaging 2016;43(1):213-219.
3. Donati OF, Chong D, Nanz D, et al. Diffusion-weighted MR imaging of upper abdominal organs: field strength and intervendor variability of apparent diffusion coefficients. Radiology 2014;270(2):454-463.
4. Keenan KE, Peskin AP, Wilmes LJ, et al. Variability and bias assessment in breast ADC measurement across multiple systems. J Magn Reson Imaging 2016;44(4):846-855.
5. Sigmund EE, Vivier PH, Sui D, et al. Intravoxel incoherent motion and diffusion-tensor imaging in renal tissue under hydration and furosemide flow challenges. Radiology 2012;263(3):758-769.
6. Friedli I, Crowe LA, Berchtold L, et al. New Magnetic Resonance Imaging Index for Renal Fibrosis Assessment: A Comparison between Diffusion-Weighted Imaging and T1 Mapping with Histological Validation. Scientific reports 2016;6:30088.
Figures