3292
Machine Learning to Identify Sarcomatoid De-Differentiation in Renal Cell Carcinoma by Multiparametric MRI1Department of Cancer Imaging & Metabolism, Moffitt Cancer Center, Tampa, FL, United States, 2Department of Diagnostic & Interventional Radiology, Moffitt Cancer Center, Tampa, FL, United States, 3Department of Anatomic Pathology, Moffitt Cancer Center, Tampa, FL, United States, 4Department of Genitourinary Oncology, Moffitt Cancer Center, Tampa, FL, United States
Synopsis
We developed a machine learning application to detect renal cell carcinoma (RCC) tumors with sarcomatoid de-differentiation, a rare form of aggressive cancer with poor prognosis. Proof-of-concept was demonstrated by analyzing multiparametric MRI volumetric data of 24 tumors, of which 11 were sarcomatoid RCC and 13 were non-sarcomatoid clear cell RCC. Our machine correctly classified 10 out of 11 sarcomatoid RCC cases (91% sensitivity) and 10 out of 13 clear cell RCC cases (77% specificity), with an overall classification accuracy of 20 out of 24 tumors (83%).
Purpose
Sarcomatoid de-differentiation of renal cell carcinoma (sRCC) is a highly aggressive form of renal cell carcinoma (RCC) associated with large size, rapid recurrence, and poor prognosis. sRCC is more responsive to chemotherapy and immunotherapy than most subtypes of RCC, and may respond better to certain systemic therapies than non sarcomatoid clear cell RCC (ccRCC) [1]. Given that suspected RCC are not routinely biopsied prior to nephrectomy, non-invasive diagnosis of sRCC will enable early stratification of patients with this uncommon subtype and potential chemotherapeutic downstaging attempts in unresectable disease. Accordingly, our goal in this study is to develop a machine learning algorithm using self-organizing map (SOM) theory [2] to differentiate sRCC from ccRCC. The SOM was trained using supervised learning on labeled sRCC and ccRCC tumor voxels in multiparametric MRI (mpMRI) scans. In the validation phase, the trained SOM was activated to classify an unknown RCC case as sRCC or ccRCC. We report the performance of our machine on the basis of sensitivity (detection of sRCC) and specificity (detection of ccRCC).Methods
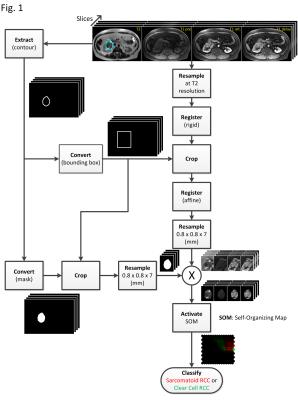
Following IRB approval, we analyzed RCC tumors in 24 patients for whom the following preoperative MRI scans were available: T2-weighted (T2w), T1-weighted (T1w: pre-contrast, arterial-phase and delayed-phase post-contrast). The RCC subtype of each tumor was confirmed during nephrectomy specimen histologic evaluation, and the presence or absence and percentage of sarcomatoid de-differentiation was quantified by a Pathologist. The sRCC cohort included 11 tumors with background histology (7 clear cell, 2 chromophobe, 2 poorly differentiated carcinoma) and the ccRCC cohort included 13 tumors. Tumors were segmented by manual contouring on each slice of the axial T2w scan by an experienced Radiologist who also confirmed the absence of significant intratumoral hemorrhage on in/out phase imaging. Our analysis comprised three main processing steps (Figure 1): registration, resampling, and classification. First, using T2w as reference, anatomical structures were spatially aligned by intensity-based registration using mutual information [3]; rigid global registration (RGR) and affine local registration (ALR) were consecutively employed. RGR provided spatial alignment at the body level to compensate for gross patient movement and differences in slice planning between sequences. ALR at the tumor level was used to correct for intra-abdominal motion. Next, all scans of all subjects were resampled to match the cohort average T2w spatial resolution to insure uniform voxel size (0.8mm x 0.8mm x 7mm). We hypothesized that such resampling would significantly improve classification performance of the SOM method by equalizing the tissue content within all voxels in the training data, thereby increasing the consistency of the mpMRI signature. The third and final processing step pertains to RCC subtype differentiation by machine learning. A total of 2 x 106 tumor voxels with known labels (ccRCC or sRCC) constituted our training set for the supervised learning phase of a SOM (225 neurons).Results
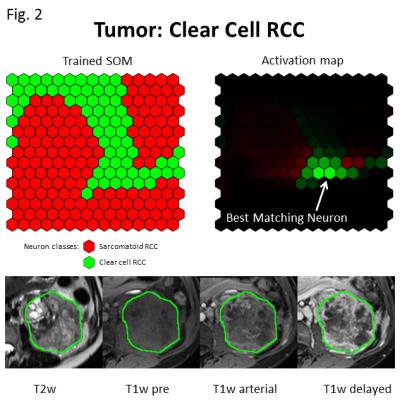
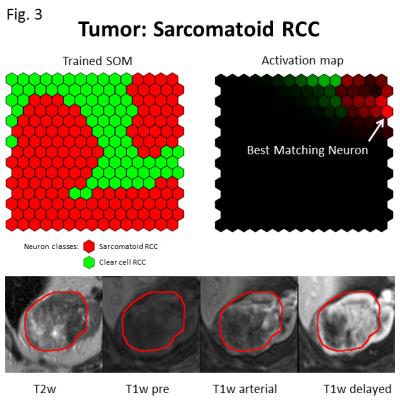
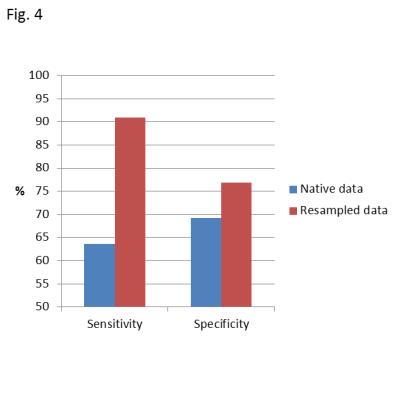
Figures 2 and 3 exemplify two representative cases of classification using a leave-one-out cross-correlation approach. In both figures, neuron brightness on the activation map indicates the number of ‘hits’, and final classification was on the basis of the most active or best matching neuron. Figure 2 depicts an example of correct ccRCC classification, with the majority of activated neurons on the activation map belonging to the ccRCC class. Figure 3 depicts an analogous example for correct sRCC classification, and while the activated area largely overlaps with neurons of the sRCC class, some neurons belonging to the ccRCC class are also activated. This may be indicative of the heterogeneous nature of sRCC tumors. Figure 4 summarizes results from a leave-one-out cross-validation of our technique, yielding a sensitivity of 91% and a specificity of 77%. Voxel resampling to equalize the volumes of tumor voxels across all patients led to a significant increase in both sensitivity (+27 percentage points) and sensitivity (+8 percentage points).Discussion
One sRCC case was misclassified by our machine, possibly because of the particularly low sarcomatoid differentiation (5%) in this tumor relative to the average (50%) of our sRCC cohort. We are performing additional studies to identify causes behind misclassification of 3 of the 13 ccRCC cases. Apparent Diffusion Coefficient (ADC) values have been shown to be lower in sRCC compared to ccRCC, but ADC maps were not uniformly available in our cohort and not included in our analysis.Conclusion
Determining presence of sarcomatoid de-differentiation features in a RCC tumor has important therapeutic implications. On a small dataset of 24 tumors, our machine learning application proved to be 91% reliable in detecting sRCC features. Our next step is to validate our machine learning algorithm on a larger dataset with incorporation of ADC.Acknowledgements
No acknowledgement found.References
[1] Takeuchi M, Kawai T, Suzuki T, et al. MRI for differentiation of renal cell carcinoma with sarcomatoid component from other renal tumor types. Abdom Imaging. 2015;40(1):112-119.
[2] Kohonen T, Self-Organized Formation of Topologically Correct Feature Maps. Biol. Cybern. 1982;43:59-69
[3] Viola P, William M. Wells I, Alignment by Maximization of Mutual Information. Int. J. Comput. Vision. 1997;24:137-154.
Figures