3290
Multiparametric MRI of renal transplant: preliminary results and repeatability study in patients with stable renal function.1Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Radiology, Groupe Hospitalier Pitié Salpêtrière, Paris, France, 3Radiology, University of Utah, Salt Lake City, UT, United States, 4Recanati Miller Transplantation Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 5Pathology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
Intrinsic conditions leading to renal graft dysfunction have so far been difficult to diagnose non-invasively because of the overlap in symptoms and laboratory metrics. MRI provides an accurate assessment of the morphology of the transplanted kidney, as well as of vascular or obstructive renal disorders. The long-term goal of our study is to validate functional MRI as a “virtual biopsy” by developing a multiparametric MRI protocol using advanced quantitative MRI sequences in renal transplant patients. We report initial results and test-retest repeatability of quantitative mpMRI parameters of diffusion, perfusion and hypoxia in renal allografts.
Purpose
Intrinsic conditions leading to renal graft dysfunction have so far been difficult to diagnose non-invasively because of the overlap in symptoms and laboratory metrics. The definitive diagnosis of renal transplant dysfunction is based on percutaneous biopsy, which is invasive, difficult to repeat and limited in sampling volume. MRI provides an accurate assessment of the morphology of the transplanted kidney, as well as of vascular or obstructive renal disorders. The long-term goal of our study is to validate multiparametric MRI (mpMRI) as a “virtual biopsy”, by developing an mpMRI protocol using advanced quantitative MRI sequences in renal transplant patients. We report early results and test-retest repeatability of quantitative mpMRI parameters of diffusion, perfusion and hypoxia in renal allografts.Methods
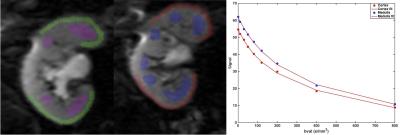
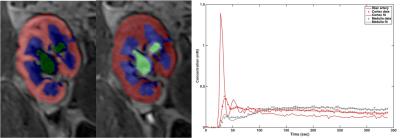
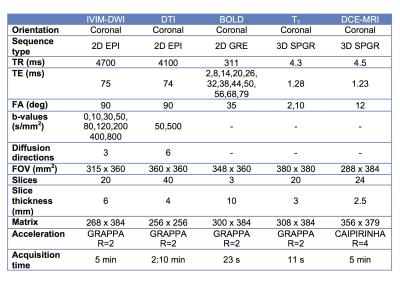
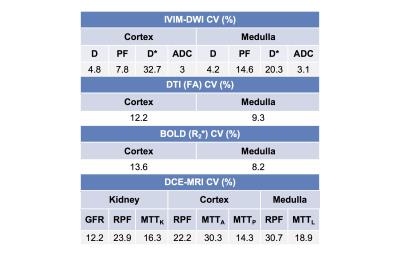
Nine initial patients (M/F 5/4, mean age 57y) including 8 with functional renal allografts (estimated MDRD serum eGFR 48-84 ml/min/1.73m2) and 1 with chronic renal dysfunction (GFR 24.6) were enrolled in this IRB-approved single center prospective study. All patients underwent mpMRI at 1.5T (Aera, Siemens) including intravoxel-incoherent motion DWI (IVIM-DWI), DTI, BOLD and DCE-MRI renography with injection of 4 ml of macrocyclic, non-ionic gadolinium agent (gadoterate meglumine, Dotarem). Acquisition parameters are displayed in Table 1. Fractional anisotropy (FA) maps were calculated on the scanner’s image reconstruction system, from the eigenvalues of the diffusion tensors. IVIM-DWI, and BOLD signal curves, as well as DTI FA values, were measured from circular ROIs placed at the upper, middle and lower renal allograft poles, using OsiriX. IVIM-DWI parameters (true diffusion D, pseudodiffusion D*, perfusion fraction PF and ADC) for the cortex and medulla were obtained by Bayesian fitting (Fig.1) from ROI-averaged signal curves1. Cortical and medullary R2* transverse relaxation rate was obtained by monoexponential fit of BOLD signal curves. Medullary-to-cortical R2* (MCR), a parameter shown to decrease in renal transplant dysfunction 2,3, was also calculated. Cortex, medulla and collecting system were segmented from DCE-MRI datasets using previously validated semi-automatic segmentation software4 (Fig.2). The iliac artery at the level of the allograft was also semi-automatically segmented to provide arterial input function. Volume-averaged signal-intensity time curves were converted to concentration-time curves using the SPGR equation, measured baseline T1 values for the renal cortex and medulla, and literature baseline T1 value for blood5. The time-concentration curves were analyzed according to a previously validated three-compartment model to extract GFR, cortical and medullary renal plasma flow (RPF) and mean transit time (MTT K) in the vascular (MTTA), proximal tubule (MTTT) and Loop of Henle compartments (MTTL) 5,6 (Fig. 2). The ratios of vascular and tubular MTT to MTTK, shown to reliably discriminate between acute rejection and acute tubular necrosis6, were calculated. Test-retest repeatability for all MRI metrics was assessed by measuring the coefficients of variation (CV) in 5 patients (average delay of 24 days between MRIs).Results
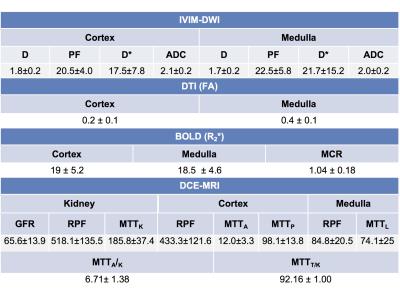
Parameter values are shown in Table 2. IVIM-DWI parameters were highly repeatable (CV <5%; Table 3), except for PF (CV cortex/medulla 7.8%/14.6%; Table 3) and D* (CV cortex/medulla 32.7%/20.3%, Table 3). R2* and FA had acceptable repeatability (CV<15%, Table 3). DCE-MRI had acceptable repeatability for GFR (CV 12.18%; Table 3), and poorer repeatability for RPF and MTT (CV 14-30%; Table 3). FA in the medulla was significantly higher compared to cortex (0.37±0.08 vs 0.18±0.06, p=0.0039, Table 2). Cortex RPF was significantly higher compared to medulla RPF (433.3±121.6 vs 84.8±20.5 ml/min, p=0.0156, Table 2). There was no significant correlation between serum eGFR and MRI parameters, between IVIM-DWI and DCE-MRI parameters, or between BOLD and DCE-MRI parameters in this early study.Discussion
Quantitative mpMRI is moderately-to-highly repeatable in renal transplants, depending on the parameter. Our DCE-MRI parameters agreed with published values in renal transplant patients with functional allografts6. IVIM-DWI and FA parameter values agreed with literature, except for D*, which is higher in our study compared to published values7. R2* values in the medulla were within the range observed by other investigators, although cortical values were higher, and MCR values were lower than previously observed in functional allografts 2,3. These discrepancies may be due to different renal transplant patient populations, and different fitting methods.Conclusions
While the quantitative MRI metrics included in our study have been individually validated in renal transplant patients, there have been no published test-retest repeatability studies in this patient population. Knowledge of test-retest repeatability would allow investigators to identify differences in mpMRI-derived parameters that reflect intrinsic renal dysfunction rather than normal physiological variation and measurement noise. The value of mpMRI-derived metrics for characterizing renal allograft dysfunction will be investigated in a larger study.Acknowledgements
We would like to acknowledge the support of the NIDDK 1 F32 DK109591-01A1 grant, the Clinical Research Center at the Icahn School of Medicine at Mount Sinai, the Morton A. Bosniak Research Award of the Society of Abdominal Radiology, and of Guerbet, LLC.References
1. Jerome NP, Orton MR, d'Arcy JA, Collins DJ, Koh DM, Leach MO. Comparison of free-breathing with navigator-controlled acquisition regimes in abdominal diffusion-weighted magnetic resonance images: Effect on ADC and IVIM statistics. Journal of magnetic resonance imaging : JMRI 2014;39(1):235-240.
2. Djamali A, Sadowski EA, Samaniego-Picota M, et al. Noninvasive assessment of early kidney allograft dysfunction by blood oxygen level-dependent magnetic resonance imaging. Transplantation 2006;82(5):621-628.
3. Han F, Xiao W, Xu Y, et al. The significance of BOLD MRI in differentiation between renal transplant rejection and acute tubular necrosis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2008;23(8):2666-2672.
4. Rusinek H, Boykov Y, Kaur M, et al. Performance of an automated segmentation algorithm for 3D MR renography. Magn Reson Med 2007;57(6):1159-1167.
5. Zhang JL, Rusinek H, Bokacheva L, et al. Functional assessment of the kidney from magnetic resonance and computed tomography renography: impulse retention approach to a multicompartment model. Magnetic Resonance in Medicine 2008;59(2):278-288.
6. Yamamoto A, Zhang JL, Rusinek H, et al. Quantitative evaluation of acute renal transplant dysfunction with low-dose three-dimensional MR renography. Radiology 2011;260(3):781-789.
7. Hueper K, Khalifa AA, Brasen JH, et al. Diffusion-Weighted imaging and diffusion tensor imaging detect delayed graft function and correlate with allograft fibrosis in patients early after kidney transplantation. Journal of magnetic resonance imaging : JMRI 2016;44(1):112-121.
Figures