3289
Reliable estimation of kidney filtration rate with DCE-MRI using motion-robust high spatiotemporal resolution Radial VIBE1Radiology, Boston Children's Hospital and Harvard Medical School, Bosotn, MA, United States, 2Radiology, Boston Children's Hospital and Harvard Medical School, Boston, MA, United States, 3Pediatrics, Boston Children's Hospital and Harvard Medical School, Boston, MA, United States, 4Surgery, Boston Children's Hospital and Harvard Medical School, Boston, MA, United States, 5Siemens Healthcare, 6Boston Children's Hospital and Harvard Medical School, Boston, MA, United States
Synopsis
Chronic kidney disease poses a significant health burden, and patients benefit from early detection of kidney function. Serum-creatinine based estimation is insensitive to early changes and nuclear medicine studies expose patients to radiation. In this work, we evaluated a novel technique, motion-robust high spatiotemporal resolution Dynamic Radial VIBE (DRV) with compressed sensing, to reconstruct high-quality dynamic image series, and to precisely estimate filtration rate per kidney and per voxel. Our results suggest that, compared to conventional Cartesian VIBE, DRV reconstructs higher quality motion-robust images and results in improved the goodness-of-fit to the tracer kinetic model, reducing RMSE and increasing the precision of filtration rate parameter.
Purpose
To evaluate if dynamic contrast enhanced (DCE)-MRI using motion-robust high spatiotemporal resolution Radial VIBE can improve estimation of kidney glomerular filtration rate (GFR) compared to conventional Cartesian VIBE in pediatric patients.Introduction
GFR is a clinically important quantitative measure of renal function. Serum creatinine based estimation is insensitive to early changes and nuclear medicine studies expose patients to radiation. DCE-MRI uses the filtration of contrast agent through the kidneys to directly measure GFR. Conventional DCE-MRI using Dynamic Cartesian VIBE (DCV) is often corrupted by respiratory and cardiac motion which reduces image quality and affects GFR estimation accuracy, especially for per voxel or per kidney segment. Another limitation is the spatial and temporal resolution required for accurate acquisition of the arterial input function (AIF) peak needed to correctly estimate GFR. Decreasing temporal resolution causes systematic underestimation of GFR. Recently, radial “stack-of–stars” sequence acquisitions have been used to reduce the effect of motion in abdominal MRI1. Radial VIBE continuously acquires radial lines including the center of k-space throughout the scan. Therefore, each sampled line contains equally important information, especially the contrast information. Balanced sampling of k-space makes the acquisition motion-robust. Compressed-sensing reconstruction of dynamic image series further improves image quality by reducing streaking artifacts2, which arise from undersampling. In this work, we assess the ability of DCE-MRI using Dynamic Radial VIBE (DRV) to obtain motion-robust images of kidneys with high spatial and temporal resolution and compare its performance for estimating filtration rate parameter to the standard DCV.Methods
We imaged six pediatric patients at 3T for six minutes after injection of Gadavist using a radial “stack-of-stars” 3D FLASH sequence (TR/TE/FA 3.56/1.39ms/12o, 32 coronal slices, voxel size=1.25x1.25x3mm). We achieved a mean temporal resolution of 3.3 sec for the arterial phase (2 minutes) and 13 sec for the remaining phases (4 minutes). 4D dynamic image series were reconstructed offline using compressed-sensing3 to improve temporal resolution and image quality, effectively reducing the streaking artifacts. We also searched the hospital database and gathered data from six patients recently imaged with the standard DCV. Using in-house software, the volumes acquired in two different phases were aligned and resampled to the same resolution. The kidney parenchyma was segmented semi-automatically. An ROI was drawn inside the aorta to determine arterial input function. The tissue enhancement curves of kidney parenchyma were converted to concentration curves and a separable two-compartment tracer kinetic model by Sourbon et al.3 was fitted to estimate the filtration rate parameter (FT). GFR is calculated by multiplying FT with renal parenchyma volume.Results
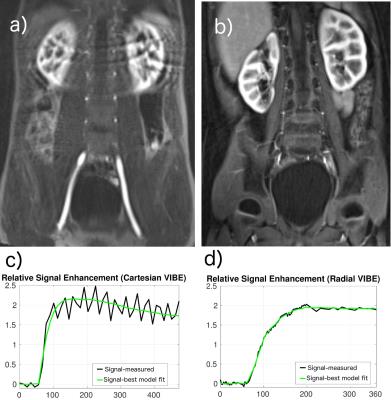
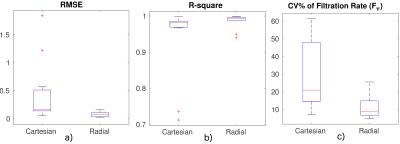
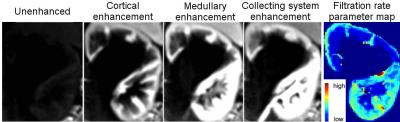
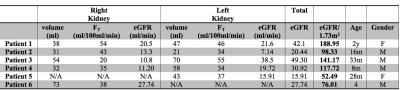
An experienced radiologist visually evaluated the DRV images (Fig.1) in six patients with various conditions affecting their kidneys. DRV consistently improved image quality by eliminating ghosting artifacts and limiting streaking artifacts when using compressed sensing reconstruction while achieving high temporal resolution (3.3sec) for all patients compared to clinical images acquired using the DCV sequence (11sec). DRV minimized the effect of motion in tissue contrast-enhancement curves (Fig.1b). Multiple high-quality post-contrast images were also generated using increased number of radial lines for additional renal morphology evaluation. We compared the goodness-of-fit of contrast-enhancement curves to the tracer kinetic model for each kidney acquired using DRV and DCV sequences using root-mean-square error (RMSE) and the R-square measures of fit (Fig.2a,b). DRV reduced the mean RMSE from 0.45±0.57 to 0.08±0.05 and increased the R-square from 0.94±0.11 to 0.99±0.02. We also used wild-bootstrap analysis4 and computed the coefficient of variation percent (CV%=standard deviation/mean x100) of filtration rate parameter (FT) as a measure of precision. DRV improved the precision of parameter estimation by reducing the average CV% from 29.84±19.72 to 10.97±6.41. In addition to per kidney FT estimation, we also estimated filtration rate parameter per voxel. A sample set of images of one patient showing different stages of contrast enhancement and the corresponding voxel-wise filtration rate parameter map is shown in Fig.3. Fig.4 reports GFR values per kidney.Conclusions
We demonstrated that DCE-MRI using DRV improved image quality, limited motion artifacts, obtained high spatial and temporal resolution and accurately acquired the AIF when compared to DCV. DRV improved the two-compartment tracer kinetic model fitting quality and improved the precision of the estimated filtration rate parameter. DRV also achieved successful computation of per voxel filtration rate parameter. Thus, DRV is potentially a useful method to estimate GFR, especially in pediatric patients for whom motion often poses as an obstacle for quality imaging. The possible use of this novel method is enormous and allows children already undergoing an MRI, such as oncology patients with impaired renal function or undergoing nephrotoxic chemotherapy, to have GFR tested simultaneously.Acknowledgements
This work is supported by the Young Investigator Award from the Society of Pediatric Radiology.References
1.Chandarana, Hersh, et al. "Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration." Investigative radiology 46.10 (2011): 648-653.
2. Feng L, Grimm R, Tobias Block K, et al. Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014;72: 707–717.
3. Sourbron, Steven P., et al. "MRI-measurement of perfusion and glomerular filtration in the human kidney with a separable compartment model." Investigative radiology 43.1 (2008): 40-48.
4. Freiman, M, Voss SD, et al. "Quantitative body DW-MRI biomarkers uncertainty estimation using unscented wild-bootstrap." In International Conference on Medical Image Computing and Computer-Assisted Intervention, pp. 74-81. Springer Berlin Heidelberg, 2011.
Figures