3286
Influence of hip position on oxygenation and perfusion of renal allografts using BOLD, DWI and ASL MRI1Depts. Radiology and Clinical Research, University Bern, Bern, Switzerland, 2Spinal cord injury Center, Balgrist University Hospital, University of Zurich, Zurich, Switzerland, 3Dept. Nephrology, Hypertension and Clinical Pharmacology, Hospital University of Bern, Bern, Switzerland
Synopsis
Functional kinking due to tethering of iliac arteries by adjacent fibrotic tissue may occur in kidney graft recipients when sitting, and in turn lead to repetitive graft hypoperfusion. The aim was to investigate if perfusion and oxygenation were changed in transplanted kidneys during leg flexion (>90°) compared to the straight-leg position by employing fMRI (DWI, BOLD, ASL). Contradicting our hypothesis, perfusion increased in flexed leg compared to straight-leg position. Furthermore, furosemide had a significantly lower impact on R2*-values in flexed than in straight leg position. In conclusion, results demonstrated an acute impact of strong leg flexion on functional renal parameters.
Background
Kidney transplantation represents the therapy of choice for patients suffering from end-stage renal disease [1]. Interstitial fibrosis/tubular atrophy determines long-term kidney graft survival and is considered multifactorial [2]. Iliac flow restrictions with kinking have been described in cyclists secondary to fibrous fixation of the iliac bifurcation [3,4]. Magnetic-resonance angiography in flexed hip position was applied to identify the kinking [3]. Functional kinking due to tethering of the iliac arteries by adjacent fibrotic tissue may also occur in graft recipients when sitting, and in turn lead to repetitive graft hypoperfusion. Therefore, the aim of this study was to investigate if perfusion and oxygenation properties are changed in kidneys of renal transplant recipients during leg flexion of >90° compared to the straight-leg position by employing fMRI (DWI, BOLD and ASL).Methods
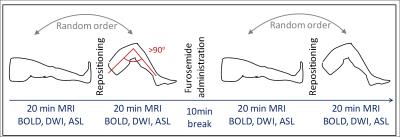
Nineteen renal transplant recipients (48±13years) with normal renal function (GFR≥30ml/min/1.73m2) were scanned on a 3T-MR Scanner (Verio, Siemens) employing the same protocol four times (acquisition time ~20min for each protocol, Fig.1), with straight and flexed legs (leg flexion angle >90°). To facilitate the >90°-flexion, measurements were performed in lateral position requiring subject positioning far off-center. Both positions were repeated after 20mg Furosemide (Lasix®) administration to account for magnetic field inhomogeneities confounding especially BOLD measurements. The positioning order (first straight then flexed, or vice versa) was changed randomly. Patients stopped diuretic medication and their hydration status was controlled. Blood samples were taken to estimate GFR.
BOLD-MRI [5-7] was performed in a single breath-hold of 17sec per slice with 12 echoes (6-52.3ms) and following parameters: TR=65ms, FOV=400x400mm2, Matrix=256x256, slice-thickness=5mm. DWI was performed with eight b-values (0-600s/mm2), 2 repetitions, TR=3300ms, TE=56ms, slice-thickness=5mm, Matrix=128x128, FOV=300x300mm2. FAIR true-fast imaging with steady-state precession (True-FISP) ASL [8] was performed (TR/TE=4.0/2.0ms, slice-thickness=7mm, Matrix=128x128, FOV=360x360mm2, TI=1200ms).
All data were analyzed using in-house custom-scripts written in IDL® and MATLAB®. Regions of interest were placed manually in cortical and medullary regions covering large parts of the kidney. DWI yielded ADCD (ADC without perfusion contribution) and perfusion fraction (FP), BOLD-MRI yielded the relaxation rate R2*, and ASL yielded perfusion-values.
Results
DWI and BOLD-MRI were successfully completed in all 19 patients and ASL measurements in 17 subjects. One patient was excluded from the analysis due to extreme outlying values (>10*sd). Overall 10% of all parameters were not obtained, either because of missing measurement or because of poor quality exclusion, based on standard criteria.
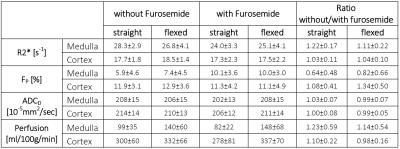
Despite the off-center patient position and the short measurement protocol, mean values and standard-deviations were similar to published values (Table 1, e.g. [6,7,9]).
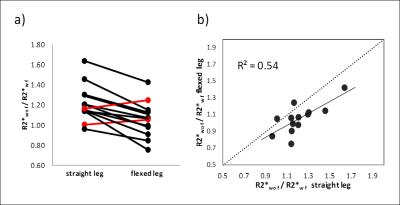
BOLD: Furosemide administration reduced medullary R2* significantly in the straight-leg position (p<0.0001). R2*-values were not significantly different between flexed and straight positions. However, the medullary R2* ratio of without/with furosemide was significantly lower in the flexed compared to the straight position (p<0.0005, Fig.2a) due to a stronger impact of furosemide on R2* in the straight-leg position. The R2* ratio was correlated between flexed and straight position (R=0.73, P<0.01, Fig.2b).
DWI: No significant difference between straight and flexed position was determined for FP and for ADCD, except for medullary ADCD which was slightly higher in the flexed than the straight position after furosemide administration. In straight-leg position, medullary FP increased significantly after furosemide application (P<0.005).
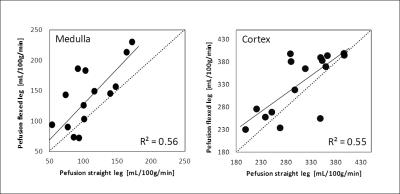
ASL: Renal perfusion-values without and with furosemide were significantly higher (P<0.03 for all) in flexed compared to straight position, except for the cortical perfusion without furosemide (P=0.051, Fig.3). In addition, perfusion-values were correlated between flexed and straight position, especially in measurements without furosemide (R=0.74, P<0.002, Fig.3). Furosemide administration led to a slight (non-significant) perfusion increase in flexed-leg position and to a perfusion decrease in straight-leg position.
Correlation coefficients between different fMRI parameters and with clinical parameters including GFR were generally low.
Discussions and Conclusion
The study demonstrates the feasibility to perform comprehensive fMRI studies in kidneys, including BOLD, DWI, and ASL with reliable results within ~20min. This short measurement duration allowed investigating the furosemide application and the measurement of two different patient positions applying three fMRI-methods in one session. Significant correlations between parameters obtained in straight and flexed leg positions as well as with and without furosemide showed the measurement stability.
Opposite to our initial hypothesis, perfusion increased in flexed leg compared to straight leg position. This result could be due to precluded blood outflow rather than the expected reduced inflow during hip flexion. However, the interpretation is speculative. Furthermore, furosemide had a significantly lower impact on R2*-values in flexed than in straight position. Thus in conclusion, the results demonstrated an acute impact of strong leg flexion on functional renal parameters.
Acknowledgements
This work was supported by the Swiss National Science Foundation (SNF) grant #320030-138150References
1. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999;341:1725-1730.
2. Pascual J, Perez-Saez MJ, Mir M, Crespo M. Chronic renal allograft injury: early detection, accurate diagnosis and management. Transplant Rev (Orlando ) 2012;26:280-290.
3. Schep G, Bender MH, van de Tempel G, Wijn PF, de Vries WR, Eikelboom BC. Detection and treatment of claudication due to functional iliac obstruction in top endurance athletes: a prospective study. Lancet 2002;359:466-473.
4. Schep G, Kaandorp DW, Bender MH, Weerdenburg H, van ES, Wijn PF. Magnetic resonance angiography used to detect kinking in the iliac arteries in endurance athletes with claudication. Physiol Meas 2001;22:475-487.
5. Prasad PV. Functional MRI of the kidney: tools for translational studies of pathophysiology of renal disease. Am J Physiol Renal Physiol 2006;290:F958-F974.
6. Thoeny HC, Zumstein D, Simon-Zoula S, Eisenberger U, De Keyzer F, Hofmann L, Vock P, Boesch C, Frey FJ, Vermathen P. Functional evaluation of transplanted kidneys with diffusion-weighted and BOLD MR imaging: Initial experience. Radiology 2006;241:812-821.
7. Seif M, Eisenberger U, Binser T, Thoeny HC, Krauer F, Rusch A, Boesch C, Vogt B, Vermathen P. Renal Blood Oxygenation Level-dependent Imaging in Longitudinal Follow-up of Donated and Remaining Kidneys. Radiology 2016;279:795-804.
8. Martirosian P, Klose U, Mader I, Schick F. FAIR true-FISP perfusion imaging of the kidneys. Magn Reson Med 2004;51:353-361.
9. Fenchel M, Martirosian P, Langanke J, Giersch J, Miller S, Stauder NI, Kramer U, Claussen CD, Schick F. Perfusion MR imaging with FAIR true FISP spin labeling in patients with and without renal artery stenosis: initial experience. Radiology 2006;238:1013-1021.
Figures