3280
Ferumoxytol Enhanced Cardiac Cine MRI in Patients with Implanted Cardiac Devices1Diagnostic Cardiovascular Imaging Laboratory, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 2Division of Cardiology, David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System, 3Department of Radiological Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States
Synopsis
With an aging population, more patients are receiving implantable cardiac devices (ICDs) annually. Many will need one or more cardiac MRI exams over the course of their lifetime. ICDs can cause artifacts and obscure evaluation of intra-cardiac morphology. While researchers have dealt with artifacts during late gadolinium enhancement imaging by using wideband inversion recovery, off resonance artifacts preclude reliable SSFP cine and SGE cine is degraded by blood saturation. We explored the use of ferumoxytol to mitigate device-related artifacts and flow limiting artifacts during cine MRI in patients with ICDs.
PURPOSE
In 2010, at least 370,000 pacemakers and 97,000 cardiac defibrillators were implanted1. Of those with cardiac devices, 75% will need at least one or more cardiac MRI over the course of their lifetime2. While researchers have explored techniques to minimize device-induced artifacts in patients undergoing MRI for fibrosis imaging3-6, the presence of artifacts on SSFP cine MRI precludes its use, and SGE cine is usually poor in the majority of these patients with impaired cardiac function. Recently, ferumoxytol (FE) has been evaluated as an off-label MRI contrast agent for bright blood imaging in a variety of conditions7. We hypothesized that the stable and specific blood pool distribution of ferumoxytol can be exploited to mitigate device-related and flow-dependent artifacts in patients with implantable cardiac devices undergoing clinical FE-MRI.METHODS
After written informed consent, cardiovascular MRI was performed at 1.5T according to established institutional protocol for patients with implantable cardiac devices. Safety protocols and medically trained personnel were present to manage any adverse events. Following routine localizers, cine gradient echo images (TR/TE 36/1.7ms, bandwidth 610 Hz, in-plane spatial resolution 1.0x2.5x6mm, FA 25°) were acquired during the steady state distribution of ferumoxytol 4 mg/kg. Image quality and artifacts were graded using a 4-point scale with a score of 4 representing excellent image quality; an image artifact score of 4 represented severe artifacts rendering the images non-diagnostic. Signal-to-noise ratios (SNR) and contrast-to-noise ratios (CNR) were quantified.RESULTS
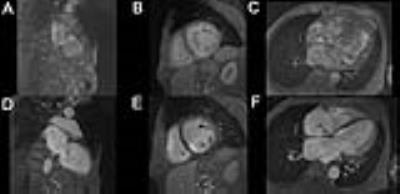
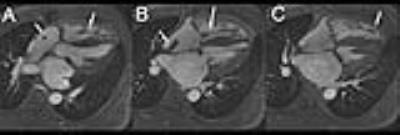
Of 285 unique patients undergoing clinically indicated FE-MRI at our institution, fourteen patients (ages 36-78 years, 3 females) had implantable cardiac devices (8 defibrillators, 6 pacemakers, 1 CardioMEMSTM) and twelve had post-FE gradient echo cine imaging. The mean estimated glomerular filtration rate was 38±20 mL/min/m2 (creatinine 2.5±2.2 mg/dL). Device manufacturers included St. Jude (n=7), Biotronik (n=2), Medtronic (n=3), and Boston Scientific (n=2). All patients underwent FE-MRI safely and without adverse events with a median follow-up time of 4.1 (IQR 1.3 to 15.7) months. There were no device-related complications acutely or at follow-up. All studies were considered diagnostic with minimal artifacts and all clinical questions for which the study was ordered were addressed. Compared to pre-FE MRI images, the average image quality score for post-FE images was higher (3.7±0.5 vs 1.3±0.6). Post-FE MRI images had lower image artifact scores (fewer artifacts) compared to pre-FE images (1.8±0.4 vs 3.6±0.6) (Figure 1-2). Four patients had additional echo imaging performed within 30 days. However, the indications for subsequent echoes were related to new symptoms and progression of disease.CONCLUSION
In patients with implantable cardiac devices requiring cardiovascular MRI, the use of ferumoxytol as a contrast agent can effectively mitigate artifacts and improve image quality for cardiac cine imaging. FE-MRI enables clear depiction of functional cardiac morphology in patients with cardiac devices.Acknowledgements
No acknowledgement found.References
1. Mozaffarian D et. al. Heart Disease and Stroke Statistics – 2016 Update. A Report from the American Heart Association. Circulation. 2016 Jan 26;133(4):e38-360.
2. Kalin R, Stanton MS. Current Clinical Issues for MRI Scanning of Pacemakers and Defibrillators. Pacing Clin Electrophysiol. 2005;28:326–328.
3. Rashid S, Rapacchi S, Vaseghi M, Tung R, Shivkumar K, Finn JP, Hu P. Improved Late Gadolinium Enhancement MR Imaging for Patients with Implanted Cardiac Devices. Radiology. 2014 Jan;270(1):269-74.
4. Rashid S, Rapacchi S, Shivkumar K, Plotnik A, Finn JP, Hu P. Modified wideband three-dimensional late gadolinium enhancement MRI for patients with implantable cardiac devices. Magn Reson Med. 2016 Feb;75(2):572-84.
5. Hong K, Jeong EK, Wall TS, Drakos SG, Kim D. Wideband arrhythmia-Insensitive-rapid (AIR) pulse sequence for cardiac T1 mapping without image artifacts induced by an implantable-cardioverter-defibrillator. 2015 Aug;74(2):336-45.
6. Shao J, Rashid S, Renella P, Nguyen KL, Hu P. Myocardial T1 mapping for patients with implanted cardiac devices using wideband inversion recovery spoiled gradient echo readout. Magn Reson Med. 2016 Mar 28.
7. Finn JP, Nguyen KL, Han F, Zhou Z, Salusky I, Ayad I, Hu P. Cardiovascular MRI with ferumoxytol. Clin Radiol. 2016 Aug;71(8):796-806.
Figures