3273
The impact of kinetic isotope effects in using deuterated glucose for metabolic experiments1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2University of Texas at Dallas
Synopsis
Deuteration of 13C-enriched glucose has been used to prolong T1 of hyperpolarized 13C with the objective of monitoring glucose metabolism to lactate. However, the effect of deuteration on the flux in glycolysis in vivo has not been investigated extensively. Deuterated glucose was studied in perfused hearts and extracts were analyzed for a deuterium kinetic isotope effect using 1H, 13C and 2H NMR spectroscopy. Unexpectedly, there was extensive exchange of methyl deuterons in lactate with aqueous medium. Perdeuteration slows metabolism of glucose to lactate.
Purpose
Metabolism of hyperpolarized [U-2H7,U-13C6]glucose to hyperpolarized [U-13C]lactate has been described in Escherichia coli (1), yeast (2), breast cancer cells (3) and lymphoma (4). Perdeuterated glucose was chosen with the intent of prolonging the 13C T1 (5). Since the percentage mass change of substituting 1H with 2H is high, reduced flux due to a kinetic isotope effect may be anticipated if a carbon-proton bond is broken along that pathway. Although individual reactions have been studied, little is known about the effect of deuteration in glucose on overall flux through glycolysis in intact tissues. It is important to understand these effects if the appearance of hyperpolarized 13C lactate is to be used as a biomarker for glucose metabolism.Methods
Metabolism of perdeuterated glucose was investigated in perfused rat hearts, n = 5 – 6 in each group. The control group was supplied with a 1:1 mixture of [U-13C6]glucose and [1,6-13C2]glucose. The methyl signal from lactate would be expected to show a 1:1 ratio of a singlet (derived from [1,6-13C2]glucose, and a doublet (derived from [U-13C6]glucose). The experimental group was perfused under identical conditions with a 1:1 mixture of [1,6-13C2]glucose plus [U-13C6, U-2H7]glucose. Metabolism of [1,6-13C2]glucose to [3-13C1]lactate was assumed constant between the two groups. Tissue extracts were analyzed by 1H, 2H and 13C NMR spectroscopy. Two biochemical processes were evaluated, a kinetic isotope effect in glycolysis and methyl proton exchange with the solvent in the alanine aminotransferase reaction.Results & Discussion
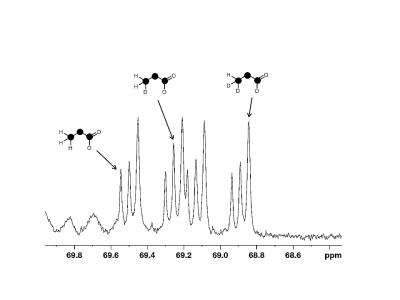
The proton-decoupled 13C NMR spectrum from control hearts showed approximately the expected 1:1 ratio of singlet to doublet in the lactate methyl group at 20.8 ppm (Figure 1, measured ratio 1:0.92 ± 0.01). The 13C NMR spectrum of metabolites derived from deuterated glucose is sensitive to both 2H-13C coupling and a chemical shift isotope effect due to deuterium. The 2H NMR spectrum of lactate methyl deuterons demonstrated 2H-13C scalar coupling of ~19.6 Hz.
Since 2H is spin 1, a single 2H bonded to 13C will generate a 1:1:1 triplet in the proton decoupled 13C NMR spectrum; two 2H coupled to a single 13C will generate a 1:2:3:2:1 quintet. The 13C NMR spectrum is also influenced by the effects of 2H-induced chemical shift of ~ 0.25 ppm for a single deuteron. Consequently, the anticipated 13C spectrum of lactate for 0, 1 or 2 methyl deuterons is easily simulated. The methyl group of pyruvate derived from perdeuterated glucose is expected to retain 2 deuterons originating from the 6 position, and 1 deuteron originating from the 1 position. Lactate derived from [U-13C6, U-2H7]glucose would be expected to show a ratio 0:1:1 forlactate with 0, 1 or 2 deuterons in the methyl group. However, a significant fraction of the lactate derived from perdeuterated glucose had no 2H in the methyl group (Figure 1).
The ratio of lactate derived from [1,6-13C2]glucose relative to lactate derived from [U-13C6, U-2H7]glucose was 1 : 0.64 (±0.13) deviating significantly from the measured ratio in control hearts, indicating that the deuteration of glucose reduced flux through glycolysis to lactate. The 13C NMR spectrum of lactate carbon 2 confirms metabolism of [U-13C6, U-2H7]glucose to [U-13C3]lactate with 0, 1 or 2 deuterons in the methyl group (Figure 2) by showing a doublet of doublets due to [U-13C3]lactate plus 2 additional signals shifted by ~0.05 and ~0.1 ppm upfield due to the chemical shift isotope effect of 1 and 2 2H in the methyl group. The spectrum indicates that methyl proton solvent exchange occurs, likely via proton exchange with solvent water at the level of alanine – pyruvate exchange. 2H enrichment in lactate methyl position was 0.55 ±0.03, 0.25±0.04 and 0.20±0.03 for 2 deuterons, 1 deuteron and 0 deuterons, respectively.
Conclusion
Perdeuteration slows metabolism of glucose to lactate. This result is consistent with the general observation that cleavage of a deuteron-carbon bond is reduced as would be expected in the conversion of 2-phosphoglycerate to phospho-enolpyruvate. This effect should must be considered in interpretation of the rate of appearance of lactate from hyperpolarized deuterated glucose.Acknowledgements
The authors would like to acknowledge the NIH (5R37-HL034557 and 8P41-EB015908) for financial support.References
1. Meier S, Jensen PR, Duus JØ. Real-time detection of central carbon metabolism in living Escherichia coli and its response to perturbations. FEBS Lett. 2011; 585: 3133-8. PMID: 21907715.
2. Meier S, Karlsson M, Jensen PR, Lerche MH, Duus JØ. Metabolic pathway visualization in living yeast by DNP-NMR. Mol Biosyst. 2011; 7: 2834-6. PMID: 21720636.
3. Harris T, Degani H, Frydman L. Hyperpolarized
13 C NMR studies of glucose metabolism in living breast cancer cell cultures.
NMR Biomed. 2013, PMID: 24115045
4. Rodrigues TB, Serrao EM, Kennedy BW, Hu DE, Kettunen MI, Brindle KM. Magnetic resonance imaging of tumor glycolysis using hyperpolarized (13)C-labeled glucose. Nat Med. 2014; 20: 93-7. PMID: 24317119
5. Keshari KR, Wilson DM. Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem Soc Rev. 2014; 43: 1627-59. PMID: 24363044; PMCID: PMC4086923.
Figures
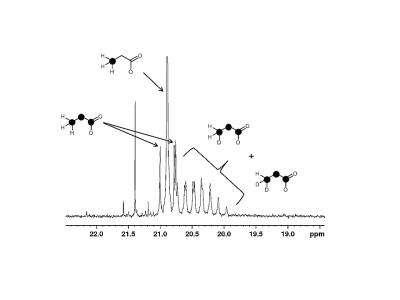
Figure 1: Proton-decoupled 13C NMR spectrum of lactate carbon 3 from hearts exposed to [1,6-13C2]glucose plus [U-13C6, U-2H7]glucose.[1,6-13C2]glucose produces [3-13C1]lactate, a singlet at ~27ppm. A black sphere marks a 13C labelled position in the structures.