3272
Novel Ultrasound Real-time Feedback Slice Tracking with Spatially Resolved MR-compatible Ultrasound for Cardio-vascular MRI1Department of Radiology, Geneva University Hospitals, Geneva, Switzerland, 2Department of Radiology, Division of Radiological Physics, University of Basel Hospital, Basel, Switzerland, 3Department of Biomedical Engineering, University of Basel, Basel, Switzerland, 4HEI, école d’Yncréa Hauts-de-France, France
Synopsis
Spatially resolved MR-compatible ultrasound was used to detect a respiratory-mimicking motion for prospective correction of cine MR data acquisitions. A scaling factor relating the motion between the two modalities was derived in real-time and was chosen to compare motion-corrected MR images to a static phantom ‘breath-hold’ and an uncorrected cine image series.
Background
Cardiac MRI has the potential to depict the cardiac valves with high resolution, to assess their functionality in terms of forward and regurgitant flow, their morphology and presence of masses, indicative of tumors or infections. However, the heart is subject to motion. We propose a method of ultrasound guidance for the compensation of motion during MR acquisition and test this principle using a ‘breathing’ phantom for high-resolution sequences that are too long for breath-holding.Methods
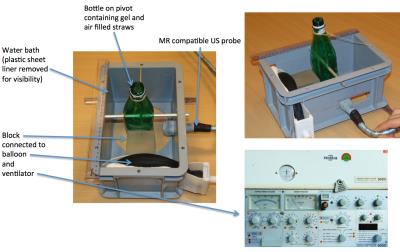
Ultrasound imaging used a clinical US system (Siemens ACUSON Antares). The P10-4 linear-array imaging probe (128-element) was modified to ensure MR compatibility, replacing magnetic materials with MR-compatible ones. An 8m long multi-channel cable was used to transfer the signals between the US probe and the control unit, placed outside the MR room. The cable was shielded against electromagnetic interference by a non-magnetic dense mesh coating set to a common ground with the Faraday cage. Additional adjustment of the impedance matching compensated for the increased capacity of the long cable. Advanced electromagnetic shielding was applied on the inner face of the transducer casting. A phantom bottle of acoustic gel, with air-filled straws inserted, was held in a water bath coupled to the probe with acoustic gel. The ventilator air output, to a balloon and block in contact with the bottle, was regulated to provide a regular breathing motion in the head-foot direction (figure 1).
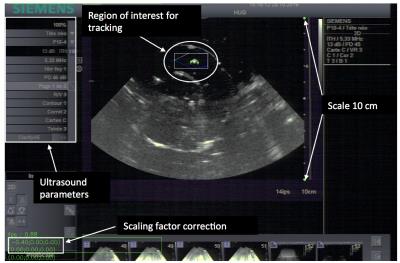
The horizontal projection of the motion vector was imaged by second harmonic ultrasonography and tracked via the acoustic speckle shift. The US field depth was 10-16cm with single-focus scanning. The frame rate was 15-30 images/second depending on field depth and angular aperture. The slice thickness was approximately 4mm (carrier frequency 4.4MHz, receive central frequency 8.8MHz). US images were sent at a frame rate of 30Hz in near real-time to an external PC, which extracted the average motion vector in-plane over a user-defined ROI and communicated the respective 2D motion vector to the MR system on-the-fly (figure 2).
A conventional cardiac-trigger segmented balanced SSFP sequence was modified in order to adapt the slice positioning in quasi-real time according to the information received from the ultrasound device. In order to allow asynchronous communication (thus avoiding the need to synchronize MR and US frame rates), a TCP server socket was implemented as a separate thread in the same MR sequence process. The server thread stored the position information (received from the US as a triplet of coordinates) in shared memory, and the sequence real-time thread updated the slice position at every cardiac phase according to the latest received coordinate packet with a scaling factor that accounts for the relative motion and the difference in imaging planes between the two modalities.
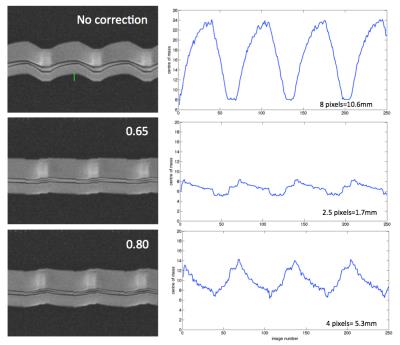
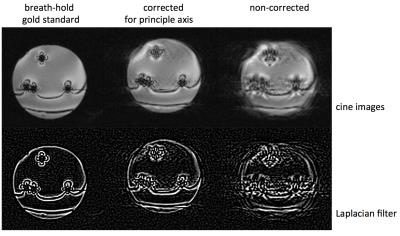
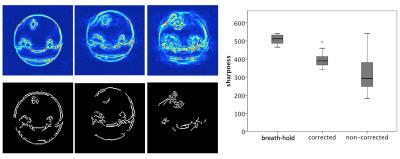
Real-time bSSFP images were acquired on a Siemens 3T PRISMA in coronal orientation (bottle cross section) with different motion-related scaling factors. The best fit was used for high-resolution cine bSSFP longer than 30s. For the real-time images, the quality of the correction is shown by calculating the centre of mass of structure in the phantom and quantifying the oscillation of this value with respect to the time course of respiration. The cine images were assessed for visual sharpness of features and can be displayed to detect edges on a Laplacian transformation. Sharpness was quantified with a Canny filter, background removed by hysteresis threshold, normalising gradient images to a binary mask, and counting white pixels at sharp edge gradients.
Results
Figure 3 shows the oscillation of time series of 250 real-time images acquired with 0, 0.65, 0.8 motion correction factors. The smaller the amplitude, the better the correction.
Cine images (>breath-hold) showed clear improvement with principle component correction (0.65 scaling) compared to uncorrected images (figure 4). The canny-filtered image showed ‘gaps’ where affected by motion artifacts (figure 5). Quantification of sharpness by Canny filter was highest for the gold standard breath-hold (for the example shown, 552 pixels), with 414 for the corrected image (243 for non-corrected). Over 18 images, quantification gave overall higher, more uniform sharpness for the corrected than the uncorrected (p=0.02, breath-hold 510±23, corrected 324±103, non-corrected 397±38).
Discussion
The required scaling factor is dependent on the relationship between the ultrasound and MR imaging planes. The differences to gold-standard are due partly to out-of-plane motion of the imaging slice (pendulum). In vivo motion will be corrected for the three orthogonal motion components.Conclusions
With this multi modality method, spatially resolved MR-compatible ultrasound was used to detect a respiratory-mimicking motion for prospective correction of cine MR data acquisitions of the principle motion component in high-resolution cine images.Acknowledgements
No acknowledgement found.References
No reference found.Figures