3270
Dynamic PET/MR Imaging of Glucose Utilization and Contractile Function Under Hypoxic Stress1Department of Pediatrics, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Medicine, University of Wisconsin-Madison, Madison, WI, United States, 3Departments of Medicine & Radiology, University of Wisconsin-Madison, Madison, WI, United States, 4Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
The purpose of this study was to establish a methodology that could be used to study the dynamic relationship between cardiac contractile function and metabolism in a serial rest-stress model using dynamic cardiac PET/MR. To assess dynamic changes in cardiac glucose metabolism we evaluated the use of a continuous infusion of 18F-FDG. We found that increases in cardiac contractility were coupled with an increase in glucose utilization in the myocardium under hypoxic stress conditions. These results demonstrate the feasibility of performing simultaneous measures of contractile function and metabolism in the heart.
Purpose
In the setting of heart disease, cardiac metabolic changes precede overt pump dysfunction1. However, in most clinical scenarios, metabolism and function are not typically assessed together. Moreover, cardiac contractile function and metabolism are typically measured under resting conditions. The purpose of this study was to develop novel methodology to study the dynamic relationship between contractile function and metabolism in a rest-stress scenario in a preclinical model. The induction of a physiological stressor, such as hypoxia or exercise, can elicit subclinical changes in cardiac function which are not typically observed under resting imaging conditions2,3. Finally, to determine the relationship between cardiac metabolism and contractile function the two must be measured together, which requires the use of simultaneous PET and MR imaging.Methods
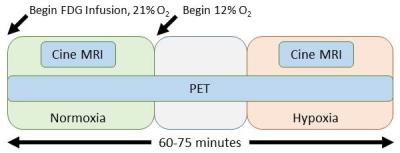
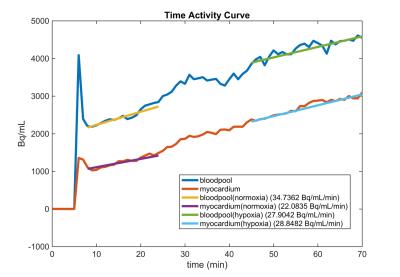
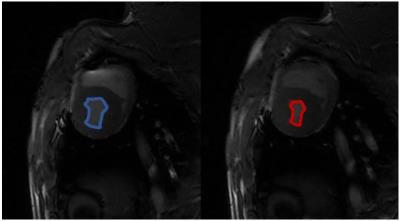
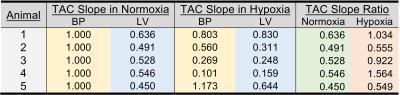
Following an overnight fast, healthy pigs (45-50kg) were anesthetized using telazol (5mg/kg)/xylazine (1mg/kg IM). An endotracheal tube was inserted and a mechanical ventilator connected to 21% oxygen, and maintenance anesthetization was maintained with isoflurane. 18F-FDG (FDG) in saline solution was administered intravenously at a constant rate of 0.01ml/s for 60 minutes using a Medrad Spectra Solaris syringe pump (initial activity ~5 mCi). For assessment of plasma glucose and lactate, arterial and venous blood samples were collected every 10 minutes from the carotid artery and external jugular vein during scanning. After 20-25 minutes of normoxic breathing at 21% oxygen, 12% oxygen gas was delivered to induce a hypoxic stress. After approximately 10 minutes of settling time to ensure a steady state hypoxic response, 20-25 minutes of continued imaging was performed, as shown in Figure 1. Dynamic, simultaneous cardiac PET/MR imaging was performed on an integrated 3T scanner (Signa PET/MR, GE Healthcare). MRI measures consisted of cardiac-gated short axis cine MR with the following parameters: 35 cm FOV, 1.3 ms TE, 3.6 ms TR, matrix 224x224, slice thickness 7cm. PET data was acquired simultaneously with MRI. Reconstruction parameters for dynamic PET are as follows: 60-75 frames of 60 second duration, 45 cm FOV, matrix 192x192, 28 subsets, 2 iterations, VPFX-S, time-of-flight, and 5 mm smoothing. Cine MR images were analyzed in Segment (Medviso, Lund, Sweden) to determine left ventricle (LV) volumes. Dynamic PET data was analyzed in custom MATLAB (The Mathworks, Natick, MA) software. Three dimensional regions of interest (ROIs) were manually drawn over the left ventricle (LV) and blood pool (BP). The respective BP and LV slopes of the time activity curve (TAC) were determined in normoxia and hypoxia as shown in Figure 2. 10 pigs were studied with MR only, and 5 pigs were studied with PET/MR. All animal procedures were performed under local ACUC approval.Results
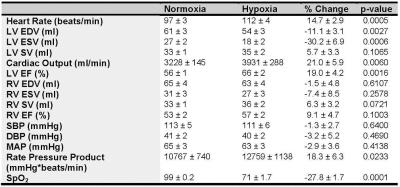
Following hypoxic stress there was an increase in heart rate (HR), cardiac output (CO), LV ejection fraction (EF), and rate pressure product (p < 0.05) as shown in Figures 3 and 4. Arterial saturation decreased from 99% to ~70% SpO2 (p<0.05) due to hypoxia exposure. There were no changes in RV volumes or RV EF. The TAC slope ratio, defined as the ratio of the LV to BP slope of the PET data increased by 74% (p<0.05) suggesting increased LV glucose utilization as a result of hypoxic stress, as shown in Figure 5.Discussion
The purpose of this study was to explore a novel methodology utilizing dynamic cardiac PET/MR techniques to simultaneous assess contractile function and metabolism in a serial rest-stress protocol. Hypoxic stress induces a significant change in contractile function as measured with MRI, which is coupled with an increased rate of LV FDG uptake relative to BP as measured with PET. Interestingly, the BP slope decreased under hypoxic stress in all but one animal, suggesting a global increase in glucose uptake. These findings are similar to what has been observed in humans hearts exposed to hypoxia4 using non-dynamic PET methods. Currently, conventional cardiac PET studies use a bolus injection of FDG to measure glucose utilization. The use of a continuous infusion of FDG allows dynamic imaging to perform a serial rest-stress protocol within a single study, which may help elucidate changes that are not measurable at rest. We have demonstrated the capability of performing this study in healthy animals, and our results suggest that these same techniques may further elucidate more subtle functional and metabolic abnormalities in the presence of heart disease. Further work to refine this methodology includes extension of the PET infusion model to full kinetic modeling5 and the integration of more advanced MR imaging such as 4D flow to assess hemodynamic and kinetic energy changes under stress.Acknowledgements
We would like to thank Dan Consigny and Sara John with their professional help on this study.References
1. Doenst, T. et al. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovascular research 86, 461-470, doi:10.1093/cvr/cvp414 (2010).
2. Boos, C. J., Mellor, A., O'Hara, J. P., Tsakirides, C. & Woods, D. R. The Effects of Sex on Cardiopulmonary Responses to Acute Normobaric Hypoxia. High Alt Med Biol 17, 108-115, doi:10.1089/ham.2015.0114 (2016).
3. Fleg, J. L. et al. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. Journal of applied physiology (Bethesda, Md. : 1985) 78, 890-900 (1995).
4. Chen, C. H. et al. Altitude hypoxia increases glucose uptake in human heart. High Alt Med Biol 10, 83-86, doi:10.1089/ham.2008.1064 (2009).
5. Hahn, A. et al. Quantification of task-specific glucose metabolism with constant infusion of 18F-FDG. Journal of nuclear medicine : official publication, Society of Nuclear Medicine, doi:10.2967/jnumed.116.176156 (2016).
Figures