3268
Interleaved 31P-MR spectroscopy and cine 1H-MR imaging of the human heart at 3 Tesla1Department of Radiology, Academic Medical Center, Amsterdam, Netherlands, 2Biomedical NMR, Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands, 3Neuroimaging Center, University Medical Center Groningen, Groningen, Netherlands, 4Department of Biomedical Engineering and Physics, Academic Medical Center, Amsterdam, Netherlands
Synopsis
Changes in the myocardial energy homeostasis have been linked to decreases in cardiac pumping performance. Current MR practice requires two consecutive recordings of 31P-MRS and 1H-MRI to assess both aspects of heart physiology, with consequently long scan times, considerable patient burden, and potentially a mismatch between data from separate sessions. By efficiently interleaving 3D ISIS acquisitions for localized 31P-MRS with short-axis cine 1H-MR imaging, both myocardial energy status as well as left-ventricular ejection fraction could be quantified from MR data that were acquired essentially simultaneously.
Purpose
This work aims to explore the feasibility of interleaved acquisitions of 3D-localized 31P-MR spectra and cine 1H-MR images of the human heart at 3 Tesla.Background
Changes in the myocardial energy homeostasis have been linked to decreases in cardiac pumping performance.1 Therefore, assessments of myocardial metabolism and function of the human heart are of clinical value. Unique to MR, it provides a noninvasive window into myocardial energy metabolism via phosphorous-31 MR spectroscopy (31P-MRS) of high-energy phosphate metabolites (phosphocreatine, PCr; ATP) as well as heart function via proton MR imaging (1H-MRI). Current MR practice requires two consecutive recordings for such measurements, with consequently long scan times, considerable patient burden, and potentially a mismatch between data from separate sessions. Particularly, these issues may be exacerbated in cardiac MR stress testing. Here, we explored the feasibility of acquiring localized 31P-MR spectra and cine 1H-MR images of the human heart in an interleaved2,3 fashion, such that both myocardial energy status and heart function can be assessed essentially simultaneously.Methods
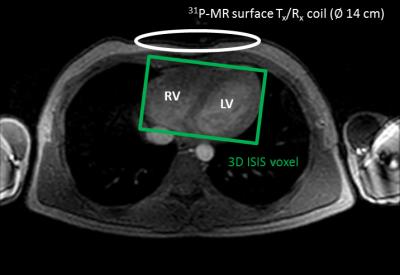
Experiments were conducted on a Philips Ingenia 3.0 Tesla MR system (Philips Medical Systems, Best, The Netherlands) equipped with a linearly-polarized 31P-MR surface transmit/receive coil (∅ 14 cm, 51.8 MHz; Philips). For shimming and 1H-MRI, the quadrature body coil was used (∅ 70 cm, 127.8 MHz). Cardiac data were acquired in a healthy male volunteer (34 y/70 kg/1.75 m) lying supine in the MR scanner with the 31P-MR surface coil carefully positioned over the heart (Figure 1).
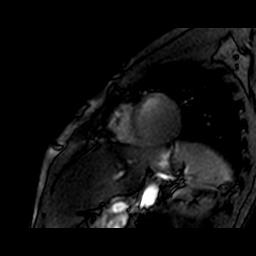
Interleaved MR acquisitions: By interleaving different scans, the idle time during long repetition times typically used in 31P-MRS can be efficiently filled with 1H-MRI acquisitions. Cardiac-triggered 3D image-selected in vivo spectroscopy (ISIS) was used to acquire localized 31P-MR spectra of the heart (voxel size 120×80×80 mm3 enclosing the left and right ventricle (Figure 1); number of averages 8 (= one 3D ISIS cycle); effective TR 9 heart beats; PCr on-resonance; bandwidth 2000 Hz; 1024 data points). During each effective TR of 9 ECG R-R intervals between the ISIS averages, a left-ventricular short-axis slice was imaged in 6 heart beats (Figure 2). These cine 1H-MRI series were acquired with a balanced steady-state free precession (bSSFP) sequence (field of view 250×320 mm2; matrix 100×128; slice thickness 10 mm; voxel size 2.5×2.5×10 mm3; TR/TE 2.4/1.22 ms; flip angle 15°; number of averages 1; 17 heart phases; retrospective ECG synchronization). Combined, a stack of short-axis cine 1H-MRI series as well as a 31P-MR spectrum were obtained from the heart within a total acquisition time of approximately one minute during a free-breathing protocol.
Data analyses: Resonance peaks in the 31P-MR spectrum were fitted to Gaussian line shapes in the time domain using AMARES in jMRUI.4 The PCr resonance was used as an internal chemical shift reference at 0.00 ppm. The γ- and α-ATP resonances were fitted with equal amplitudes and line widths within each doublet and a J-coupling constant of 17 Hz. Left-ventricular ejection fraction was quantified by manual segmentation of the endocardial left-ventricular wall in the 1H-MR images using Segment.5
Results
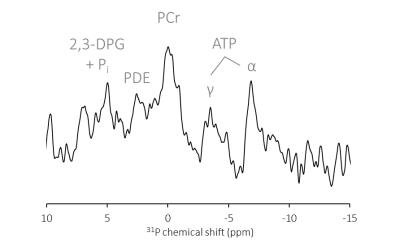
Figure 3 shows the single-voxel 3D ISIS-localized 31P-MR spectrum obtained from the heart of a healthy volunteer with one 3D ISIS cycle. Although the signal-to-noise ratio is low, resonance peaks from the myocardial high-energy phosphate metabolites PCr and ATP and erythrocyte 2,3-diphosphoglycerate (2,3-DPG) that are characteristic of cardiac 31P-MR spectra can be distinguished. Myocardial energy status, quantified via the PCr/γ-ATP ratio, was 2.70. During the TR idle time window of 3D ISIS acquisitions, short-axis cine 1H-MR images were acquired (Figure 4). Left-ventricular ejection fraction was 56.3%.Discussion
Until now, cardiac MR investigations have been limited to either 31P-MRS or cine 1H-MR imaging in a consecutive protocol. Here, we demonstrated the feasibility of acquiring localized 31P-MR spectra and cine 1H-MR images of the human heart in an interleaved fashion within a one-minute free-breathing protocol. At this time, low signal-to-noise ratios and relatively poor image quality hamper any diagnostic interpretation of the data. However, ongoing developments in MR hardware6 and RF coil design7 are expected to improve data quality, enabling essentially simultaneous assessments of heart function and myocardial energy status with this protocol of interleaved acquisitions. In particular, the approach presented here would greatly benefit dynamic MR studies of the human heart, e.g., during exercise or adenosine stress.8Acknowledgements
This work was supported by the National Institutes of Health (NIH grant HL072011).References
1. Tewari SG, et al. J Mol Cell Cardiol 2016;94:162-75
2. Meyerspeer M, et al. Magn Reson Med 2007;57;654-60
3. Henningsson M, et al. Magn Reson Med 2015;73:692-96
4. Vanhamme L, et al. J Magn Reson 1997;129:35-43
5. Heiberg E, et al. BMC Med Imaging 2010;10:1
6. Löring J, et al. NMR Biomed 2016;29:709-20
7. Bakermans AJ, et al. Proc ISMRM 2016;24:3518
8. Dass S, et al. Eur Heart J 2015;36:1547-54
Figures