3267
Contact- free diaphragm navigation using the scatter of a parallel transmit coil at 7T1Oxford Centre for Clinical Magnetic Resonance Research (OCMR), University of Oxford, Oxford, United Kingdom
Synopsis
A method is presented to measure respiratory motion in mm units based on the scatter of a parallel transmit coil. The respiratory changes in scatter are observed by the local SAR monitor and converted into a respiratory measure through a calibration phase. The newly described measure was compared to the diaphragm position measured in MR images. The standard deviation of the difference between the two was 1.1±0.4 mm across six volunteers. The method was implemented into a free breathing cardiac cine which showed no respiratory artefact when used to gate.
Introduction
Respiratory motion remains a limiting factor to extending the acquisition time of cardiac MRI that is often needed to increase the spatial resolution. Several navigation methods have been proposed(1–5). We present an alternative method that is contact-free, has no sequence design constraints or additional hardware and provides the diaphragm position in mm units. It uses RF safety measurements on a parallel transmit system (pTx)(6). pTx RF coils are an N-Port network where N is the number of transmit channels. The combination of coil design and complex permittivities of the subject’s tissue determine the scatter (S) matrix, which can be measured by monitoring the RF with directional couplers (DICO’s) on each of the coils. During respiration the lungs complex permittivity changes(7) due to changing air content and movement of the organs changes the load of the network.Methods
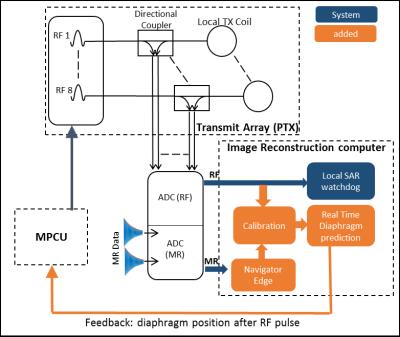
A Siemens 7T MRI scanner with pTx and local SAR monitor (VB17-step-2.3), and local transmit array(8,9). The system measures the forward and returned RF voltage for each transmit line as depicted in Figure 1. The vector $$$\overrightarrow{V}_{ret}(t)$$$ describes the complex returned RF voltages and can be modelled as a function of the expected scatter-matrix $$${\bf S}_{exp}$$$ (NxN, which is measured prior to scanning), the change in $$${\bf S}$$$ due to respiration $$$∆{\bf S}_{motion}(t)$$$, and the measured forward voltage $$$[\overrightarrow{A}⨀\overrightarrow{V}_{fwd}(t)]$$$ which is an element wise multiplication the B1+ shim ($$$\overrightarrow{A}$$$) and forward voltage of the amplifier $$$\overrightarrow{V}_{fwd}(t)$$$ as shown in equation 1.
$$\overrightarrow{V}_{ret}(t) = ({\bf S}_{exp}+∆{\bf S}_{motion}(t))[\overrightarrow{A}⨀\overrightarrow{V}_{fwd}(t)]$$[1]
For a single B1+ shim we cannot solve for $$$∆{\bf S}_{motion}(t)$$$, however we can determine a vector $$$\overrightarrow{Γ}(t)$$$ that is a function of $$$∆{\bf S}_{motion}(t)$$$, using an element wise complex division of the returned voltage resulting from $$${\bf S}_{exp}$$$ as follows:
$$\overrightarrow{Γ}(t) = \overrightarrow{V}_{ret}(t)\oslash({\bf S}_{exp}[\overrightarrow{A}⨀\overrightarrow{V}_{fwd}(t)])$$[2]
The complex vector $$$\overrightarrow{Γ}(t)$$$ is determined from data sampled over the central 50µs of each RF pulse ($$$k$$$) and split into its real and imaginary components, giving a 16 element vector $$$\overrightarrow{Γ}'_{k}$$$. Finally, a mixing vector $$$\overrightarrow{m}$$$ and constant offset $$$c$$$ are determined using a calibration cycle where the diaphragm position is measured for 30s with MRI and is recorded. $$$\overrightarrow{m}$$$ and $$$c$$$ are determined by minimising the difference between $$$d_{k}$$$ and the diaphragm position (linear regression).
$$d_{k} = {Γ}'_{k}\overrightarrow{m} + c$$[3]
Seven healthy male volunteers were recruited according to our institutions ethical practices. A sagittal spoiled gradient echo (SPGR) image was setup through the right hemi-diaphragm from which both a diaphragm image and $$$\overrightarrow{Γ}'_{k}$$$ can be determined. The SPGR had a flip angle ~5°, TR/TE: 5/2 ms, GRAPPA of 2 and 6:8 phase partial Fourier, matrix size of 304x156, FOV of 300x300 mm, duration 322 ms, repeated for 2:45s (513 measurements). The first 30s were used for calibration of $$$\overrightarrow{m}$$$ and the remaining 2:15 s for evaluation. For assessment the diaphragm position measured in the MR images is up sampled to the RF pulse rate.
To evaluate the method a pulse sequence and image reconstruction program was setup to perform the calibration using a diaphragm navigator(10). The sequence performed prospective respiratory gating and cardiac synchronisation in real-time for each RF pulse as depicted in Figure.1. A free-breathing cardiac cine SPGR was setup with a 1x1x3 mm resolution with a ±3 mm acceptance window.
Results
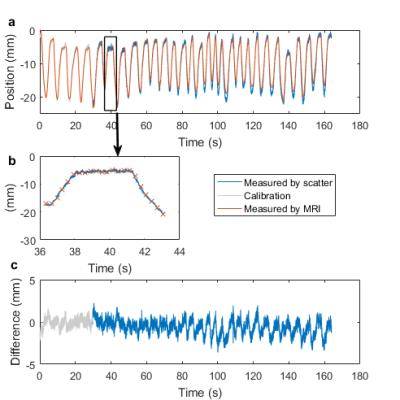
The measured diaphragm position from one subjects is shown in figure 2. in six of the subjects the standard deviation of the difference to the diaphragm position in the image was 1.1±0.4mm. The standard deviation of the discrete differentiation of this difference was calculated and divided by √2 giving 0.4±0.1mm. In one of the subjects a 20mm drift was observed from the start of the scan to the end, along with a 40% deviation in reflected voltage compared to that expected by $$${\bf S}_{exp}$$$ indicating a change in the system.
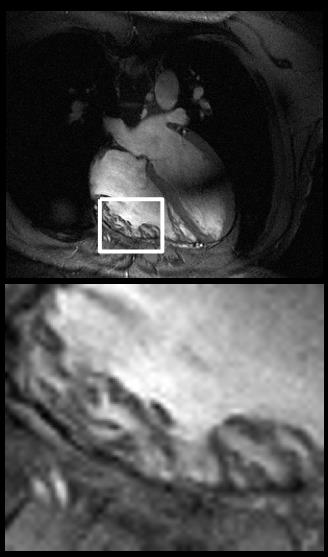
Figure 4 shows a single time frame from the free breathing cardiac cine.
Discussion
A method to rapidly determine the diaphragm position for TR-to-TR respiratory gating has been presented. The use of the s-matrix for detecting changes in the position of the internal organs is a promising new approach for quantitative real-time motion correction. The approach is not yet perfect, but these systematic differences were small, and enabled the acquisition of free breathing cine which was respiratory artefact free. The method should be calibrated for each B1+ shim used as can be appreciated by $$$\overrightarrow{A}$$$ in the equations.Conclusion
A method to provide rapid diaphragm position has been presented and evaluated that is contact- and hardware-free. Once calibrated the diaphragm position can be determined rapidly during a single excitation pulse.Acknowledgements
The British heart foundation (BHF) centre for research excellence. Medical Research Council UK.References
1. Paling MR, Brookeman JR. Respiration artifacts in MR imaging: reduction by breath holding. J. Comput. Assist. Tomogr. 10:1080–2.
2. Wang Y, Riederer SJ, Ehman RL. Respiratory Motion of the Heart: Kinematics and the Implications for the Spatial Resolution in Coronary Imaging. Magn. Reson. Med. 1995;33:713–719. doi: 10.1002/mrm.1910330517.
3. Wang Y, Rossman PJ, Grimm RC, Riederer SJ, Ehman RL. Navigator-echo-based real-time respiratory gating and triggering for reduction of respiration effects in three-dimensional coronary MR angiography. Radiology 1996;198:55–60. doi: 10.1148/radiology.198.1.8539406.
4. Cheng JY, Alley MT, Cunningham CH, Vasanawala SS, Pauly JM, Lustig M. Nonrigid motion correction in 3D using autofocusing with localized linear translations. Magn. Reson. Med. 2012;68:1785–97. doi: 10.1002/mrm.24189.
5. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn. Reson. Med. 2015;0:n/a-n/a. doi: 10.1002/mrm.25665.
6. Hess A, Rodgers C, Robson M. Real-time diaphragm navigation using reflected power measurements from a multiple channel transmit RF coil on a human 7T. In: 24th meeting of the International socieity for magnetic resonance in medcine. Singapore; 2016. p. 3309.
7. Nopp P, Rapp E, Pfutzner H, et al. Dielectric properties of lung tissue as a function of air content. Phys. Med. Biol. 1993;38:699–716. doi: 10.1088/0031-9155/38/6/005.
8. Snyder CJ, DelaBarre L, Metzger GJ, van de Moortele P-F, Akgun C, Ugurbil K, Vaughan JT. Initial results of cardiac imaging at 7 Tesla. Magn. Reson. Med. 2009;61:517–24. doi: 10.1002/mrm.21895.
9. Hess AT, Bissell MM, Ntusi NAB, et al. Aortic 4D flow: Quantification of signal-to-noise ratio as a function of field strength and contrast enhancement for 1.5T, 3T, and 7T. Magn. Reson. Med. 2015;73. doi: 10.1002/mrm.25317.
10. Hess AT, van der Kouwe AJ, Tisdall MD, Neubauer S, Robson MD. 2D Diaphragm navigation with rapid gradient echo images: validation at 3T and application at 7T. In: ISMRM 23rd Annual Meeting & Exhibition. Vol. 0. ; 2015. p. 2565. doi: 10.1002/mrm.25262.
Figures