3262
Contact-free Cardiac Motion Estimation using the Scatter of a Parallel Transmit Coil at 7T MRI1Oxford Centre for Clinical Magnetic Resonance Research, University of Oxford, Oxford, United Kingdom, 2Oxford Centre for Functional MRI of the Brain, University of Oxford, United Kingdom
Synopsis
Cardiovascular MRI at ultra-high field strength is a growing field of research and as such there is a need for reliable, accurate, and user-independent triggering and gating. We propose a novel approach that uses directional couplers from the SAR monitoring system and an 8-channel pTx coil to measure the reflection coefficients simultaneously to determine cardiac motion with an independent component analysis and Gaussian shaped RF-monitoring pulses. This approach has the potential to improve cardiac imaging at ultra-high field CMR by providing robust triggering in periods of breath-hold and free-breathing, additional gating information and an optimised workflow with no additional set-up.
Purpose
The purpose of this work is to explore
new cardiac motion estimations, to interrogate the feature space and the ability
to use RF-scatter to gate cardiac acquisitions.Methods
Previous work on RF reflection was used to estimate motion using pick up coils or additional RF sources1–4. We propose a method that uses an 8-channel transmit RF coil with separate RF amplifiers and directional couplers (DICO) on each of these transmit channels to explore whether the scattering matrix (S-matrix) measurements from these DICO’s can be used to determine cardiac motion with an independent component analysis (ICA). The returned voltages are a function of reflection from the subject and coupling through the subject from other transmitters. The S-Matrix detects if the objects and their complex permittivity change within these RF fields.
$$$S_{i,j} = \frac{ V_{i,j,Return}}{V_{j,incident}}$$$
A 7T MRI Scanner (VB17, Step 2.3, Siemens 7.0 T Magnetom scanner, Erlangen, Germany), 8-channel PTx system with local SAR monitor and an 8 channel TEM cardiac transmit/receive coil was used with four elements placed anterior on the chest of the subject and four elements placed posterior5. S-matrix measurements were made using a frequency multiplexed (2kHz channel spacing) 5ms, monitoring Gauss RF pulse every TR=10ms in 6 healthy adults (Male, age= 24-39 years) during a 15s breath-hold followed by free-breathing for 25s.
Data pre-processing
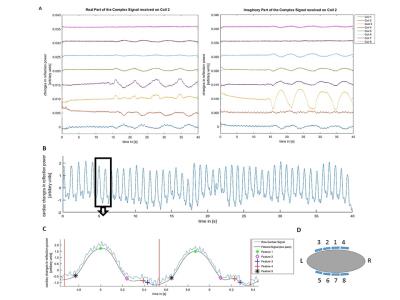
The time series of the 8x8 S-matrix was split into real and imaginary components, providing 128 observations per time point. The data was temporally de-trended and the cardiac signal was extracted using an ICA (FastICA6 for Matlab, Version 2.5) of cardiac band-pass filtered data. The cardiac component was identified by a Welch power spectrum density estimate with highest power in the respective cardiac frequency band (f= 0.6-2.4Hz).The ICA de-mixing vector was used to establish a raw, unfiltered scalar signal as shown in Figure 1.
Cardiac Signal Analysis and Feature Detection
5 features were identified and assessed in a retrospective signal analysis to detect different phases of the heart cycle. A main peak detection (feature 1), the start of the diastasis when the heart is almost at rest (feature 2), two for the start of atrial contraction (feature 3 and 4) and one to detect the start of ventricular contraction (feature 5). Using the unfiltered and de-mixed cardiac signal three further traces were established to identify the features. The first is derived from a discrete wavelet transform (DWT) with a maximal overlap. A mother wavelet with N=5 vanishing moments of the family of symlets was used and the dominate heart frequency DWT and peak detection was carried out. The other two are the first and second derivatives derived from a low pass filtered signal to identify periods of rest and high velocity in the cardiac cycle.
Results
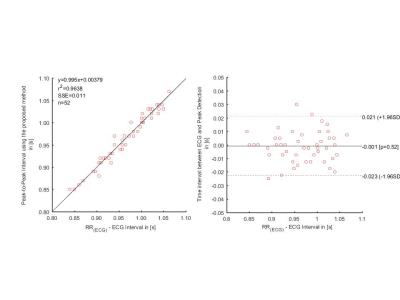
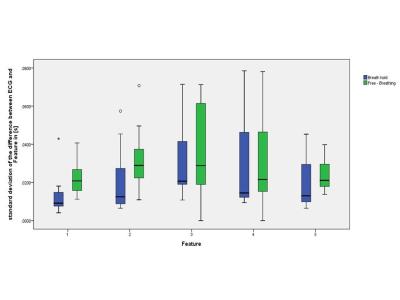
Using the ECG as a gold standard, the sensitivity and positive predictive value of the feature 1 detection was 100%, the mean of the standard deviation of the difference in trigger time between ECG and the new method was 12.5ms during breath-holding and 22ms while free breathing with a mean trigger delay of 302ms and 260ms respectively. The Bland-Altman analysis demonstrated that the RR-interval was within 21ms of that calculated with ECG on a 96% confidence interval (see Figure 2). The standard deviation of the difference in trigger time of the other features are shown in Figure 3.Discussion
The proposed method can achieve an automated, retrospective estimation of cardiac motion using a full scattering matrix derived from reflected power measurements with the commercial SAR-monitor of a 7T MRI. We showed that the cardiac motion can be estimated in-vivo during breath hold as well as in free breathing using an ICA. Feature 3 and 4, which aim to detect the atrial contraction (corresponding P-wave in the ECG), don’t provide a reliable detection when changes due to atrial contraction were around the noise floor of the data, resulting in a lower sensitivity and positive predictive value in some subjects. Feature 2, which aims to detect the start of the diastasis, shows a good overall performance and can provide additional information compared to ECG and its single peak detection. Only in higher mean heart rates is a lower sensitivity (mean Se= 88%) observed which gives rise to a reduced duration of the resting state of the heart and therefore a smaller change in the refilling.Conclusion
A new
method for cardiac motion estimation in MRI has been proposed that can be
rolled out to PTx cardiac MRI scanners assessing different stages of the heart
movement.Acknowledgements
This work was supported by funding from the Engineering and Physical Sciences Research Council (EPSRC) and Medical Research Council (MRC) [grant number EP/L016052/1], the Clarendon Fund and Keble College de Breyne Scholarship.References
1. Buikman, D., Helzel, T. & Roeschmann, P. The rf coil as a sensitive motion detector for magnetic resonance imaging. Magn. Reson. Imaging 6, 281–289 (1988).
2. Graesslin, I. et al. Advancements in Contact-free Respiration Monitoring using RF Pick-up coils. Proc. 18th Sci. Meet. Int. Soc. Magn. Reson. Med. 3045 (2010).
3. Graesslin, I. et al. An Alternative Concept for Non-Sequence Interfering, Contact-free Respiration Monitoring. Proc. 17th Sci. Meet. Int. Soc. Magn. Reson. Med. Honolulu, 752 (2009).
4. Guido P. Kudielka, Christopher J. Hardy, Pierre-André Vuissoz, Jacques Felblinger, A. C. S. B. Utilization of the receive coil for cardiovascular and respiratory motion representation. in Proc. Intl. Soc. Mag. Reson. Med. 23 (2015) 0705. 2, 2015 (2015).
5. Snyder, C. J. et al. Comparison between eight- and sixteen-channel TEM transceive arrays for body imaging at 7 T. Magn. Reson. Med. 67, 954–964 (2012)
6. Gävert, H., Hurri, J., Särelä, J. & Hyvärinen, A. FastICA package for MATLAB. (2005)
Figures