3260
Characterizing the Cardiac Phase Dependence of Motion Compensated CODE cDTI1Department of Radiological Sciences, University of California, Los Angeles, CA, United States, 2Biomedical Physics IDP, University of California, Los Angeles, CA, United States, 3Department of Bioengineering, University of California, Los Angeles, CA, United States
Synopsis
First and second order motion compensated convex optimized diffusion encoding (CODE-M1M2) enables robust, high resolution cardiac diffusion tensor imaging (cDTI). However, timing of the diffusion encoding relative to the cardiac cycle still requires careful evaluation to achieve precise and accurate measurements. In this study, CODE-M1M2 cDTI was acquired in healthy volunteers at both mid-systole and diastole to identify differences in fiber orientation, fiber cone of uncertainty (CoU), mean diffusivity (MD) and fractional anisotropy (FA). While fiber orientations were equivalent, lower CoU, lower MD, and higher FA were observed in mid-systole than in diastasis indicating improved performance.
Introduction
Cardiac diffusion tensor imaging (cDTI) with first and second order motion compensation (M1M2) enables high quality mapping of myocardial fiber orientations in vivo [1, 2]. The Convex Optimized Diffusion Encoding (CODE) technique designs diffusion gradient waveforms that are M1 and M2 compensated (CODE-M1M2) to achieve the shortest TEs and consequently higher SNR for high-resolution spin echo EPI (SE-EPI) cDTI [3].
A key consideration for cDTI is the timing of the diffusion gradients relative to the cardiac cycle. Diastasis tends to have less apparent motion and may accommodate a longer diffusion encoding and longer imaging temporal footprint without bulk motion artifacts or motion blurring [2, 4]. Several recent studies, however, have somewhat surprisingly demonstrated that M1M2 cDTI is more robust to motion when acquired in mid-systole [1, 3, 5].
The objective of this study was to characterize the differences in fiber orientation, fiber Cone of Uncertainty (CoU) [6], mean diffusivity (MD) and fractional anisotropy (FA) between mid-systolic and diastolic CODE-M1M2 cDTI in healthy volunteers.
Methods
Healthy volunteers (N=10) were imaged on a 3T scanner (Siemens Prisma) in an IRB approved study. Free-breathing CODE-M1M2 SE-EPI cDTI was navigator triggered to end expiration with b=0 and 350s/mm2, 12 directions, 2.0x2.0x5.0mm resolution and 5 averages. Imaging was performed in both mid-systole (100ms ECG trigger delay) and late diastole (subject-specific ECG trigger delay determined from bSSFP cines) in a single mid-ventricular short-axis slice. Each acquisition was repeated twice to facilitate bootstrapped precision analysis (scan time: ~20 minutes).
Diffusion tensors were reconstructed for each phase from the first 5-average set using linear least squares. Left ventricular (LV) helix angles (HA) were calculated pixel-wise and binned transmurally by percent wall depth in 10% bins.
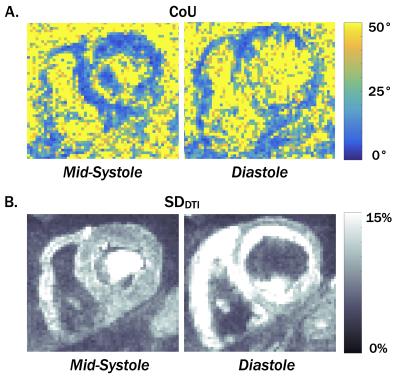
Fiber orientation precision was measured at each voxel using the cone of uncertainty (CoU) [6]. Briefly, cDTI data for each pixel were randomly sampled from the two repeated acquisitions, the tensor was reconstructed, and the process was repeated 1000 times (for each pixel). The CoU was measured within each pixel as the 95% confidence interval of the angle between primary eigenvectors and the dyadic mean of the 1000 tensors. The median CoU in the LV was measured for each volunteer and was compared between systole and diastole. Median MD and FA values in the LV were also measured from the first 5-average set.
The standard deviation of the image intensity across all 10 repetitions of each DTI direction (SDDTI) was measured at each voxel and normalized by mean signal intensity to evaluate the signal variance of mid-systole compared to diastole. The median myocardial SDDTI was compared between mid-systole and diastole.
Results
Fiber orientations and HA maps from mid-systolic and diastolic cDTI were qualitatively in agreement (Fig. 1A-B). No apparent differences in HA distributions were observed between phases (Fig. 1C).
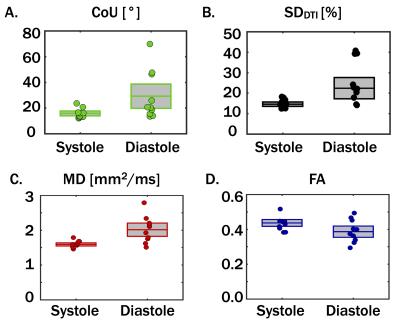
Maps of fiber orientation precision (CoU) and SDDTI are shown for one subject in Fig. 2. Median myocardial CoU, SDDTI, MD, and FA values are plotted for all 10 subjects in Fig. 3. The median myocardial CoU was significantly lower in systole than in diastole (15.8±3.7° vs. 29.2±18.9°, p=0.04, Fig. 3). Median ADC was significantly lower and less variable in mid-systole (1.61±0.09mm2/ms vs. 2.00±0.38mm2/ms, p=5x10-3) whereas FA was slightly higher and less variable in mid-systole (0.43±0.04 vs. 0.39±0.06, p=N.S.). Median myocardial SDDTI was significantly lower in mid-systole than in diastole (14.9±2.1% vs. 25.5±10.4%, p=0.01).
Discussion
M1M2 compensation dramatically reduces sensitivity to bulk cardiac motion in SE-EPI cDTI. The results of this study, however, indicate that M1M2 compensation does not fully correct for subtle and unpredictable diastolic motion. Our findings are, however, in line with previous studies [1, 3, 5] which showed excellent robustness at mid-systole.
Across the ten subjects, diastolic cDTI reported both a higher mean CoU and greater inter-subject CoU variability than mid-systolic cDTI. Underlying the larger CoU during diastasis is an elevated SDDTI, which indicates larger intravoxel signal variance that likely arises from subtle bulk motion irregularities during this phase. The elevated SDDTI, confers signal variance that that is consistent with both the elevated ADC and decreased FA.
Conclusion
CODE-M1M2 cDTI demonstrated reduced bulk motion sensitivity in mid-systole compared to diastole which resulted in higher fiber orientation precision, lower MD and higher FA in healthy volunteers.Acknowledgements
Funding support from the Department of Radiological Sciences, NIH R01HL131975, and the American Heart Association predoctoral fellowship.References
1. Stoeck CT, von Deuster C, Genet M, Atkinson D, Kozerke S. Second-order motion-compensated spin echo diffusion tensor imaging of the human heart. Magn Reson Med. 2015.
2. Nguyen C, Fan Z, Xie Y, Pang J, Speier P, Bi X, et al. In vivo diffusion-tensor MRI of the human heart on a 3 tesla clinical scanner: An optimized second order (M2) motion compensated diffusion-preparation approach. Magn Reson Med. 2016.
3. Aliotta E, Wu HH, Ennis D. Convex Optimized Diffusion Encoding (CODE) Gradient Waveforms for Minimum Echo Time and Bulk Motion Compensated Diffusion Weighted MRI. Magnetic Resonance in Medicine. 2016.
4. Nguyen C, Fan Z, Sharif B, He Y, Dharmakumar R, Berman DS, et al. In vivo three-dimensional high resolution cardiac diffusion-weighted MRI: A motion compensated diffusion-prepared balanced steady-state free precession approach. Magn Reson Med. 2013;72(5):1257-67.
5. Welsh C, Di Bella E, Hsu E. Higher-Order Motion-Compensation for In Vivo Cardiac Diffusion Tensor Imaging in Rats. IEEE Trans Med Imaging. 2015.
6. Jones DK. Determining and visualizing uncertainty in estimates of fiber orientation from diffusion tensor MRI. Magn Reson Med. 2003;49(1):7-12.
Figures