3257
Validation of Contrast Enhanced Cine Steady-State Free Precession and T2-Weigthed CMR for Assessment of Ischemic Myocardial Area-At-Risk1The MR Research Centre, Aarhus University, 8200, Denmark, 2Danish Diabetes Academy, Odense, Denmark, 3Department of Cardiothoracic and Vascular Surgery T, Aarhus University Hospital, 8200, Denmark, 4Department of Endocrinology and Internal Medicine, Aarhus University Hospital THG, Denmark, 5Department of Cardiology, Aarhus University Hospital, 8200, Denmark
Synopsis
Measuring myocardial salvage is important to evaluate the possible cardioprotective effects of adjunctive cardioprotective intervention in patients with myocardial infarction undergoing primary percutaneous intervention. Contrast-enhanced steady-state free precession magnetic resonance imaging (CE-CINE) has recently been used to quantify AAR and validated against myocardial perfusion SPECT. In this study we sought to determine how well T2-STIR and CE-CINE depicts AAR in an experimental porcine model of myocardial ischemia-reperfusion injury using histopathology as the reference for infarct size and AAR.
Background
Measuring myocardial salvage is crucial to quantify the potential cardioprotective efficacy of adjunctive cardioprotective intervention in patients with myocardial infarction undergoing primary percutaneous intervention1 . Myocardial salvage is defined as the difference between the myocardial Area-At-Risk (AAR) and final infarct size. More recently attention has been focused on determining the integrity of the microvasculature to ensure myocardial perfusion during reperfusion2. The underlying variables can in theory be determined by a single comprehensive cardiovascular magnetic resonance (CMR) examination. Measuring myocardial AAR by CMR using T2 weighted imaging for detection of myocardial edema has been challenging and major controversy questions the validity of T2 weighted CMR for quantifying AAR3. Thus, recent data from an experimental study suggest that the bright signal on T2 weighted imaging reflects myocardial necrosis rather that edema4. Furthermore, in infarcts with reperfusion injury expressing microvascular obstruction and hemorrhage, the hyperintensive signal delineated from T2 weighted imaging in the presence of myocardial edema is subtle. In addition, a bimodal pattern of myocardial edema during the first week after ischemia/reperfusion has been demonstrated in experimental studies indicating that edema appears abruptly upon reperfusion, dissipates at 24 h and reappears with maximum day 7 after reperfusion5. More recently, contrast-enhanced steady-state free precession magnetic resonance imaging (CE-CINE) has been used to quantify AAR and validated against myocardial perfusion SPECT6,7. We sought to determine how well T2-STIR and CE-CINE depicts AAR in an experimental porcine model of myocardial ischemia-reperfusion injury using histopathology as the reference for infarct size and AAR.Methods
Eleven female Danish domestic pigs weighing 40 kg were used for the experiments. The pigs were anaesthetized with sevoflurane and mechanically ventilated. Coronary occlusion was induced by placing a 2.5 mm angioplasty balloon in the LAD distal to the second diagonal branch artery and inflating it to 10 atm. The balloon occluded the LAD for 65 minutes. At the end of the experiment waited an average of 8.5 days before CMR imaging, harvesting, and histopathology was performed. CMR was performed on a 1.5 T Philips Achieva dStream (Philips Medical Systems, Best, The Netherlands). Left ventricular (LV) function was assessed using a retrospective, ECG-triggered steady-state free precession breath-hold cine sequence in the cardiac short-axis, vertical long axis and horizontal long axis planes. T2-STIR fast spin echo (TR 2400 ms; TE 100 ms; echo train length 20; fat inversion time 180 ms; flip angle 90°; 0.54 mm x 0.54 mm in-plane; averages 2; slice thickness 8 mm; FOV 320 mm x 320 mm; 14 slices) and CE-CINE magnetic resonance imaging was performed to measure AAR (TR 3.0 ms; TE 1.5 ms; FA 60°; 30 heart phases; 1.22 mm x 1.22 mm in-plane; slice thickness 8 mm; FOV of 288 mm x 288 mm; 14 slices). Furthermore, late gadolinium contrast enhancement (LGE) was performed to estimate final infarct size (TR 5.78 ms; TE 2.78 ms; echo train length 20; inversion time ~320 ms; flip angle 25°). After constriction of the occlusion position a solution of 10% Evans blue dye was injected into the left auricle to delineate AAR. The heart was then cut into consecutive 8 mm-thick and then stained with 2 % triphenyltetrazolium chloride (TTC) solution to delineate the infarct (infarcted, yellowish-white; noninfarcted, brick-red). One observer, blinded to the distribution of the groups, analysed all the CMR images using the semi-automatic software Segment version 2.0 R5165.Results
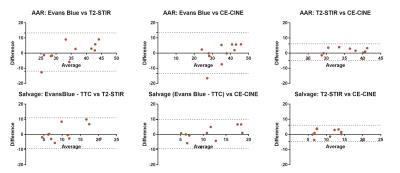
Physiological values (mean/SD) obtained during CMR were: heart rate 57±13 min-1; LV endsystolic volume 49±18 mL; LV enddiastolic volume 95±21 mL; stroke volume 45±10 mL, LV ejection fraction 49±10 %. AAR for T2-STIR was 34.5±5.5%, for CE-CINE 35.2±6.3% and for Evans blue 35.3±9.7%. Salvage for T2-STIR was 10.5±5.0%, for CE-CINE 11.2±4.3% and for [Evans blue – TTC] 11.3±6.7%. Infarct size for TTC was 24.0±6.4% and for LGE 24.0±4.6%. All infarcts demonstrated microvascular obstruction on LGE images. Bland-Altman plots showed were no significant difference in AAR or myocardial salvage between T2-STIR and CE-CINE or between CMR and histopathology (Figure 1). The limits of agreements(95%) were: AAR: Evans Blue vs T2-STIR[-11.9;13.3]; AAR: Evans Blue vs CE-CINE[-13.4;13.5]; AAR: T2-STIR vs CE-CINE[-4.8;6.1]; Salvage: Evans Blue - TTC vs T2-STIR[-9.3;10.9]; Salvage: Evans Blue - TTC vs CE-CINE[-9.4;9.7]; Salvage: CE-CINE vs T2-STIR[-4.7;6.1]. Representative matched cross-sectional images with the delineated infarct and AAR are shown in Figure 2.Conclusion
Both T2-STIR and CE-CINE sequences allows quantification of AAR in the presence of ischemia/reperfusion injury with microvascular obstruction. These experimental data, which was validated by histopathology, supports the use of CMR for the assessment of myocardial salvage.Acknowledgements
Funded by The Danish Diabetes Academy supported by the Novo Nordisk Foundation.References
1. Botker HE, Kaltoft AK, Pedersen SF, Kim WY. Measuring myocardial salvage. Cardiovascular research. 2012;94(2):266-275.
2. Wuest W, Lell M, May M, et al. Determining Microvascular Obstruction and Infarct Size with Steady-State Free Precession Imaging Cardiac MRI. PloS one. 2015;10(3):e0119788.
3. Croisille P, Kim HW, Kim RJ. Controversies in cardiovascular MR imaging: T2-weighted imaging should not be used to delineate the area at risk in ischemic myocardial injury. Radiology. 2012;265(1):12-22.
4. Kim HW, Van Assche L, Jennings RB, et al. Relationship of T2-Weighted MRI Myocardial Hyperintensity and the Ischemic Area-At-Risk. Circ Res. 2015;117(3):254-265.
5. Fernandez-Jimenez R, Sanchez-Gonzalez J, Aguero J, et al. Myocardial edema after ischemia/reperfusion is not stable and follows a bimodal pattern: imaging and histological tissue characterization. J Am Coll Cardiol. 2015;65(4):315-323.
6. Sorensson P, Heiberg E, Saleh N, et al. Assessment of myocardium at risk with contrast enhanced steady-state free precession cine cardiovascular magnetic resonance compared to single-photon emission computed tomography. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2010;12:25.
7. Kumar A, Beohar N, Arumana JM, et al. CMR imaging of edema in myocardial infarction using cine balanced steady-state free precession. JACC. Cardiovascular imaging. 2011;4(12):1265-1273.