3256
Quantitative Inversion Time Prescription for Late Gadolinium Enhancement Using T1-based Synthetic Inversion Recovery Imaging – Eliminating the Subjective Estimation of Inversion Time1Department of Radiology and Radiological Science, Medical University of South Carolina, Charleston, SC, United States, 2Department of Radiology, Leiden University Medical Center, Leiden, Netherlands
Synopsis
Conventional Look-Locker (LL)-based inversion time (TI) estimation prior to late gadolinium enhancement (LGE) imaging has multiple limitations, including: the long breath-hold, the collected images are in different cardiac phases, and the subjective TI estimation. In this study we aimed to develop a quantitative T1 mapping-based synthetic inversion recovery (IRsynth) approach allowing for the quantitative determination of the optimal TI for LGE imaging. We showed in 40 patients that the IRsynth method provides better quality of myocardial signal nulling, retrospective TI selection, higher TI resolution, no need for further LL correction or TI adjustment, and less operator dependence.
Purpose
Conventional Look-Locker (LL)-based estimation of the optimal inversion time (TI) (“TI-scout”) is a requirement prior to late gadolinium enhancement (LGE) imaging to achieve the appropriate image contrast especially when LGE images are acquired in a magnitude fashion. The TI-scout sequence has multiple limitations, including: the long breath-hold necessary, the collected images are in different cardiac phases, and the overly subjective and user-dependent TI estimation. Therefore, in this study we aimed to develop and test a quantitative T1-mapping-based synthetic inversion recovery (IRsynth) approach1,2 allowing for the calculation of the most optimal TI for LGE imaging.Methods
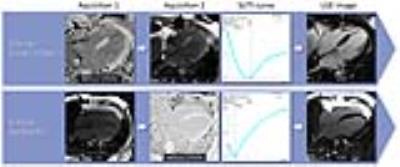
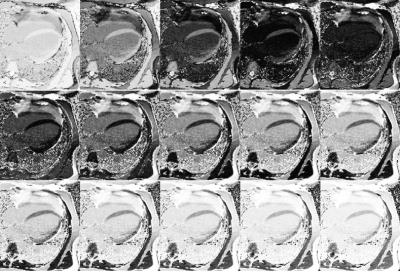
Forty consecutive patients (57±15 years, 23 male) referred for myocardial viability evaluation underwent cardiovascular MRI on a 1.5T system (MAGNETOM Avanto, Siemens, Erlangen, Germany). Twelve minutes after contrast administration (0.1mmol/kg gadobenate-dimeglumine) conventional TI-scout (LL, Field-of-view 300×256mm2; slice thickness 8mm; acquisition matrix 192×104; spatial resolution 2.08×2.08mm2; TR/TE 2.5/1.1ms; Bandwidth 965Hz/pixel; and Flip angle 50°) and T1-mapping (modified LL IR; scheme 4(1)3(1)2; Field-of-view 300×256mm2; slice thickness 8mm; acquisition matrix 196×128; spatial resolution 1.56×1.56mm2; TR/TE 2.6/1.1ms; Bandwidth 1085Hz/pixel; and Flip angle 35°) acquisitions were performed in the 4-chamber view. LL and T1 image sets were acquired in a random order and 2 subgroups were defined accordingly (Figure 1). In the LL group, T1-mapping was performed first followed by the conventional LL acquisition. In the Synthetic group, conventional LL was obtained before T1 mapping. In both groups the second acquisition was used to estimate the optimal TI for LGE imaging in order to reduce the time between the image set used for TI calculation and the LGE acquisition. Based on the T1-maps, magnitude IRsynth images were calculated in a TI range of 200-400 with 5ms increments real-time on the scanner (Figure 2). The optimal TI was determined from both LL and IRsynth images based on TI-Signal intensity curves (Figure 1). Image quality including the quality of nulling, the ability to differentiate LGE from blood, and the ability to differentiate LGE from normal myocardium was subjectively rated by two observers on a 3-point Likert scale. The quality of nulling was also objectively measured based on the myocardial/background signal ratio in the LGE images. The two groups were compared using the Kruskal-Wallis and Mann-Whitney tests.Results
Out of the 40 patients enrolled in the study, LGE consistent with myocardial infarct was observed in 21 (52.5%) subjects. The optimal TI was measured significantly lower by the conventional LL approach compared to the IRsynth technique (LL group: 259±50ms vs. 290±53ms, P<0.0218; Synthetic group: 248±21ms vs. 287±29ms, P<0.0001). The acquisition order did not influence the value of optimal TI (LL group P=0.2540; Synthetic group P=0.8760). There was significant difference between the TI estimated based on the LL acquisition, and the TI actually applied for the LGE scan (259±50s vs. 285±52ms, P=0.0021) due to the need to account for LL correction (“fudge factor”). However, there was no difference between the TI estimated based on the IRsynth image sets and the TI used for the LGE scans. Using the IRsynth-based TI for LGE acquisition (n=21) provided significantly higher image quality ratings compared to LL-based LGE (n=19) for the quality of nulling (2.4 [2.0-2.7] vs. 1.8 [1.4-2.3], P=0.0044), however there was no difference in the ability to differentiate LGE from blood (2.5 [2.2-2.8] vs. 2.6 [2.1-3.0], P=0.2415) or LGE from normal myocardium (2.7 [2.3-3.0] vs. 2.5 [2.3-2.7], P=0.1194). Myocardial/background signal intensity ratio was lower in the Synthetic group compared to the LL group (1.2±0.4 vs. 2.4±1.1, P=0.0079).Discussion
Our results indicate that T1-mapping based IRsynth imaging has the potential to accurately measure the optimal TI for LGE imaging. Subjective and objective image quality analysis showed that using the TI accurately determined based on the IRsynth image set yields better quality of nulling. The IRsynth method has multiple further advantages over the conventional LL approach. The IRsynth technique provides higher TI resolution as images can be generated at even 1ms intervals. This technique depends less on the operator as the actual TI used for LGE imaging can be retrospectively selected. Drawing regions of interest is more straightforward as all the images in an IRsynth set are in the same cardiac phase. Further advantage also reducing the subjectivity of the technique is that LL correction is already built in the T1-fitting algorithm3, thus there is no need to TI adjustment with the IRsynth technique, contrary to the conventional LL technique.Conclusion
T1-based IRsynth imaging provides objective, quantitative, and real-time prescription of the optimal TI for LGE imaging; eliminating the need for LL correction and the substantial operator dependence of the acquisition.Acknowledgements
No acknowledgement found.References
1. Xue H, Shah S, Greiser A, et al. Motion correction for myocardial T1 mapping using image registration with synthetic image estimation. Magn Reson Med 2012;67(6):1644-1655.
2. Varga-Szemes A, van der Geest RJ, Spottiswoode BS, et al. Myocardial Late Gadolinium Enhancement: Accuracy of T1 Mapping-based Synthetic Inversion-Recovery Imaging. Radiology 2016;278(2):374-382.
3. Kellman P, Herzka DA, Hansen MS. Adiabatic inversion pulses for myocardial T1 mapping. Magn Reson Med 2014;71(4):1428-1434.
Figures