3246
Optimized 2D radial CAIPIRINHA for cardiac perfusion MRI1Physics and Astronomy, University of Utah, Salt Lake City, UT, United States, 2Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States
Synopsis
We propose an optimized offset golden angle trajectory of 3-slice radial CAIPI for more uniform distribution of phase modulated rays. Both computer simulation and phantom scan demonstrate that our proposed method has less artifact. Simulation studies show a 41% reduction in aliasing energy when compared to conventional golden angle trajectory. As well, optimization with an asymmetric echo readout resulted in a reduction of acquisition time by 15% with little sacrifice of image quality. These two methods can benefit simultaneous multi-slice perfusion acquisitions.
Introduction
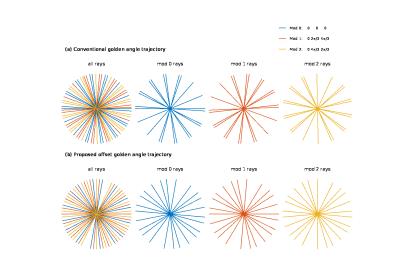
Controlled aliasing in parallel imaging result in higher acceleration (CAIPIRINHA or CAIPI) method applies different phase modulations on all of the slices that are stimulated simultaneously to reduce aliasing energy [1]. Radial golden angle (GA) sampling trajectory yields flexibilities of choosing an arbitrary window length and window position for reconstruction[2], thus the combination of radial CAIPI with GA trajectory is favorable for cardiac perfusion studies[3]. For 3-slice CAIPI, using conventional golden angle trajectory leads to a non-uniform distribution of rays with different phase modulation modes in k-space, shown in Fig 1. Wu et. al. proposed a modified GA sampling trajectory with an azimuthal angle increment of $$$ 111.246°/N $$$ for multi-band factor of $$$ N $$$ to solve this problem[4]. With our proposed offset GA trajectory, a more uniform distribution of rays with different phase modulation modes is achieved and the aliasing artifact is further reduced. In addition, we study the use of asymmetric echo readouts to reduce TE and readout time for radial CAIPI.Methods
In conventional radial GA sampling trajectory, the k-space data is acquired along each ray at angle $$$0, \alpha, 2\alpha, 3\alpha ...$$$ where $$$\alpha$$$ is the golden angle ($$$111.246°$$$). The proposed method (offset GA) is to sample the k-space at angles $$$0-\beta, 0, 0+\beta, \alpha-\beta, \alpha, \alpha+\beta, 2\alpha-\beta, 2\alpha, 2\alpha+\beta…$$$ with $$$\beta$$$ a fixed angle chosen based on the number of rays used for reconstruction ($$$\beta = \pi/n$$$ with $$$n \leq$$$ number of rays used for reconstruction).
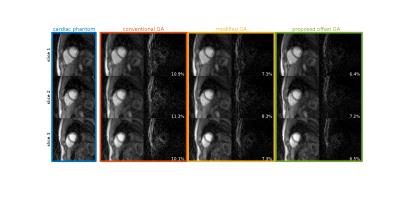
Simulation Study 30 frames of dynamic cardiac perfusion images were used as our test phantom with 3 slices for each frame, and three different methods were compared: 1) conventional GA trajectory, 2) modified GA trajectory as in [4], 3) offset GA trajectory with $$$\beta = \pi/36$$$. Each image was projected on 36 rays in k-space according to the azimuthal angles above using non uniform fast Fourier transform (NUFFT). Then the k-space data was phase modulated and the 3 slices were combined to create simulated k-space data. 4 channels were assumed with different coil sensitivities. Reconstruction was done using NUFFT and a multi-coil spatiotemporal constrained reconstruction (STCR) framework[5]. For each method, simulated images without CAIPI were also acquired for comparison.
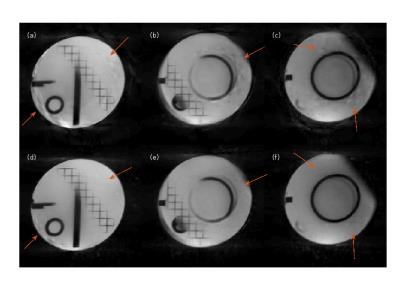
Phantom Study Phantom images were acquired using both conventional GA and offset GA with $$$\beta = \pi/24$$$ trajectories on a 3T Prisma (Siemens) scanner, with a radial turboFLASH sequence of parameters TR/TE = 2.4/1.3 ms, FOV = 260mm and voxel size 1.8×1.8×7mm. 24 rays were acquired for each time frame over 180 degrees. Images were reconstructed using NUFFT STCR.
Another optimization, to study asymmetric echo readout, was performed. An asymmetry metric was defined as $$$100 \% \times (A + B)/2B$$$, where $$$A$$$ and $$$B$$$ are durations of the acquisition window before and after the echo center.
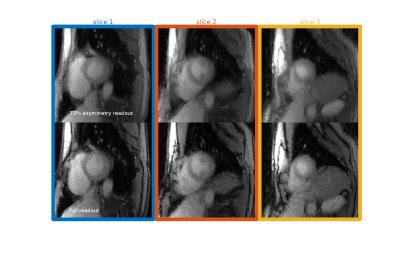
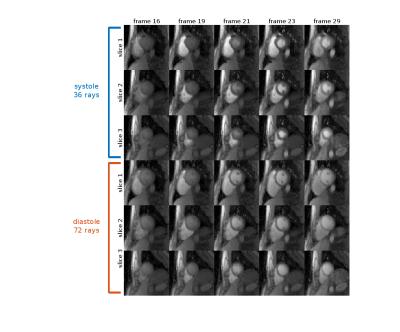
Human Study Tests without an associated contrast injection were run on one subject on a 3T Prisma scanner with TR/TE = (2.2/1.0) for 73% asymmetry and TR/TE = (2.6/1.4) for full readout, FOV = 260mm and voxel size 1.2×1.2×10mm. Perfusion images with a contrast injection were also acquired on the same subject with 73% asymmetry readout. Rays were acquired over 360 degrees. 36/72 rays were chosen from systole/diastole cycle to reconstruct each time frame using NUFFT STCR.
Results and Conclusion
Simulation study results are shown in Fig 2. The aliasing energy ratio was defined as $$$100\% \times \|I_d\|_1/\|I_0\|_1$$$, where the $$$I_d$$$ is the difference from non-CAIPI images, $$$I_0$$$ is the reconstructed image with CAIPI and $$$\| \|_1$$$ stands for the $$$l_1$$$ norm. The proposed method yields a least aliasing energy ratio at 4.1% for an average of 3 slices and 30 frames, comparing to 4.4% for modified GA and 6.9% for conventional GA. Fig 3 shows the phantom scan images, where the proposed method leads to visibly less artifact. Asymmetric echo readout images are shown in Fig 4 and Fig 5, which have acceptable quality for quantization studies. With 73% asymmetry readout, a reduction of 0.4 ms in TR can be achieved, which leads to ~15% more rapid acquisition and higher temporal resolution.
The proposed offset GA phase modulation method has the least aliasing artifact compared to conventional GA and modified GA, and with asymmetric readout data can be acquired more rapidly. These two methods can benefit simultaneous multi-slice perfusion acquisitions. More experiments on both methods and their combination are desired to test their utility in practice.
Acknowledgements
No acknowledgement found.References
[1] Yutzy et al. MRM 65(6): 1630-1637, 2011 [2] Winkelmann et al. IEEE-TMI 26:68-76, 2007 [3] Wang et al. MRI 34(9): 1329-1336, 2016 [4] Wu et al. JMRI 44:158-167, 2016 [5] Ganesh et al. JMRI 29(2): 466-473, 2009
Figures