3244
10-fold Spatial-Only Acceleration For High-Resolution Myocardial Perfusion Using Multi-Band Imaging and Multi-Band Outer Volume Suppression1Electrical and Computer Engineering, University of Minnesota, Minneapolis, MN, United States, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3Computer Assisted Clinical Medicine, Heidelberg University, Mannheim, Germany, 4Department of Cardiology, University of Minnesota, Minneapolis, MN, United States
Synopsis
Myocardial perfusion imaging is clinically established for the detection of myocardial ischemia and requires rapid imaging to monitor the uptake of a contrast-agent in the heart. Spatial resolution or coverage is commonly increased by exploiting temporal correlations, at the risk of inducing temporal blurring. Here, we investigate the use of simulteaneous multi-slice imaging for high spatial-only acceleration. Outer-volume-suppression using multi-band saturation-slabs (MB-OVS) were used to facilitate high multi-band factors. Phantom results, show through signal suppression outside region-of-interest with MB-OVS. In-vivo results show robust image quality throughout the contrast uptake and washout with 9-slice LV coverage at a temporal resolution <550ms.
Introduction
First pass myocardial perfusion imaging is the clinical gold-standard for detection of myocardial ischemia1,2. It is commonly performed with two-to-four short-axis slices acquired every RR interval with T1-weighted contrast using saturation-recovery3. However, limited left-ventricular coverage and coarse spatio-temporal resolutions are main factors compromising diagnostic image quality. To improve this, highly-undersampled 2D or 3D imaging techniques have been proposed, often relying on the utilization of spatio-temporal correlations4,5. This necessitates modeling of the temporal dynamics, breath-holding or motion correction. An alternative is to use simultaneous multi-slice or multiband (MB) imaging to acquire multiple slices simultaneously6, a recently proposed acceleration technique that shows minimal loss in the imaging SNR if appropriate coil geometries are provided7. The SNR reduction and leakage artifacts in cardiac imaging may be mitigated by suppression of extra-cardiac tissue. Such suppression8 has recently shown potential in 3D stack-of-spirals perfusion imaging9. In this study, we sought to investigate the potential of MB acceleration with a MB outer-volume suppression (MB-OVS) in whole-heart high-resolution myocardial perfusion imaging with a spatially-regularized non-linear reconstruction algorithm.Methods
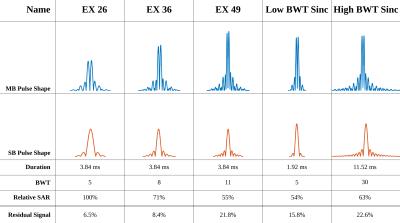
Perfusion imaging was performed at three cardiac phases in the proposed sequence (Fig. 1), each following a non-selective saturation and a short saturation-delay. FLASH imaging with linear reordering is employed for data acquisition. Simultaneous multi-band excitation of three slices was performed with RF phase-cycling7. MB-OVS pulses are played directly prior to and interleaved with the imaging pulses, to obtain optimal suppression of extra-cardiac tissue suppression. MB-OVS consists of a slab-selective 90° pulse to two slabs (on chest and back) followed by 200µs gradient spoiling. Multiple RF pulse shapes (Fig. 2) were investigated for MB-OVS saturation. For both MB-OVS and MB excitation pulses, constant phase shifts were added to each band to minimize B1+ peak amplitude.
Imaging was performed at 3T (Siemens Magnetom Prisma). Phantom images were acquired to confirm the efficiency of MB-OVS using various RF pulses. In-vivo first-pass perfusion imaging was performed with injection of 0.05 mmol/kg gadobutrol (Gadovist) at 4 mL/s, followed by a 10-mL saline flush. 3-fold MB acceleration, 3-fold uniform in-plane acceleration, and partial-Fourier=7/8 were utilized for an overall 10-fold acceleration. MB-OVS preparation was interleaved between every 9 imaging pulses. The imaging parameters were: FOV=320x320mm2, resolution=1.7x1.7mm2, slice-thickness=8mm, TR/TE/FA=2.9/1.7ms/12°, temporal resolution=163ms, saturation time=150ms. 3 separate MB slice-stacks were acquired within each heart-beat, for a total of 9-slice coverage. A 1-second reference low-resolution scan (6x6 mm2) of 3 slices and at 3 cardiac phases, during free-breathing, was used to generate coil sensitivity profiles. Iterative non-linear reconstruction was performed for each MB slice using a B1-weighted approach with in-plane TV-regularization.
Results
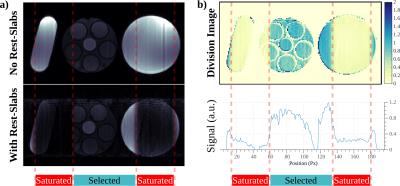
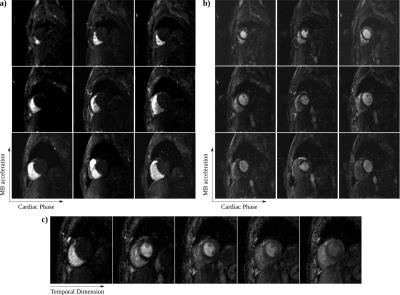
The performance of different pulse shapes are shown in Fig. 2, with EX 36 chosen as a trade-off between suppression efficiency, SAR and slab-profile, and the associated MB-OVS takes 3.45ms. Fig. 3 depicts the phantom results for the effectiveness of MB-OVS preparation in the perfusion sequence for a single-slice acquisition, showing good signal suppression outside the region-of-interest. Fig. 4 shows representative reconstructed images from a healthy volunteer using the proposed MB acquisition with MB-OVS. Temporal dynamics corresponding to the contrast arrival in the RV (Fig. 4a) and after contrast arrival in the LV (Fig. 4b) show good separation of MB slices and limited artifacts due to the high in-plane acceleration. Note that the groups of 3 slices are at different cardiac phases due to sequence design. Fig. 4c depicts a mid-ventricular slice across multiple temporal dynamics, showing no signification variation in quality through time.Disucssion
The proposed technique enables a 10-fold acceleration acquisition of myocardial perfusion images with 9-slice coverage, 1.7mm in-plane resolution, and a 163ms temporal resolution, suggesting the total acquisition can be confined to <500ms. Furthermore, the reconstruction technique only relies on spatial information, avoiding issues with temporal blurring or need for motion compensation. Additional improvements in reconstruction quality and higher acceleration rates may be possible using more advanced regularization techniques10.
MB processing of Cartesian data has a simple characterization in terms of FOV/MBFactor shifts in image space. Thus, the use of OVS has an intuitive interpretation, in removing extra-cardiac structure for reduced fold-over artifacts due to slice acceleration. While this also enables a simple reconstruction framework, the linear ordering used in 2D Cartesian acquisitions necessitates interleaving of the OVS pulses, limiting their duration. To obtain good signal suppression at a minimal pulse duration we used an MB approach to excite multiple saturation slabs simultaneously. This allowed its interleaving into the acquisition pulse train, while avoiding contrast disruption.
Acknowledgements
Funding: NIH R00HL111410, NIH P41EB015894.References
1. Nagel E, Klein C, Paetsch I, Hettwer S, Schnackenburg B, Wegscheider K, Fleck E. "Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease." Circ 2003;108:432–437
2. Schwitter J, Nanz D, Kneifel S, Bertschinger K, Buchi M, Knusel PR, Marincek B, Luscher TF, von Schulthess GK. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation 2001;103(18):2230-5.
3. Gebker R, Schwitter J, Fleck E, Nagel E. "How we perform myocardial perfusion with cardiovascular magnetic resonance." J Cardiovasc Magn Reson. 2007;9(3):539-47.
4. Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med 2010;64:767–776.
5. Vitanis V, Manka R, Giese D, Pedersen H, Plein S, Boesiger P, Kozerke S. "High resolution three-dimensional cardiac perfusion imaging using compartment-based k-t principal component analysis." Magn Reson Med 2011;65:575–587
6. Stäb D, Wech T, Breuer FA, Weng AM, Ritter CO, Hahn D, Köstler H. "High resolution myocardial first-pass perfusion imaging with extended anatomic coverage." J Magn Reson Imaging. 2014 Jun;39(6):1575-87.
7. Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. "Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging." Magn Reson Med. 2005 Mar;53(3):684-91.
8. Smith TB, Nayak KS. "Reduced field of view MRI with rapid, B1-robust outer volume suppression." Magn Reson Med. 2012 May;67(5):1316-23.
9. Yang Yang, Li Zhao, Xiao Chen, Kelvin Chow, Peter W Shaw, Jorge A Gonzalez, Frederick H Epstein, Craig H Meyer, Christopher M Kramer and Salerno M. "Reduced field-of-view stack-of-spirals enables high spatiotemporal resolution 3D perfusion imaging" J Cardiov Magn Reson 2016;18(Suppl 1):P325
10. Akçakaya M, Basha TA, Goddu B, Goepfert LA, Kissinger KV, Tarokh V, Manning WJ, Nezafat R. "Low-dimensional-structure self-learning and thresholding: regularization beyond compressed sensing for MRI reconstruction." Magn Reson Med. 2011 Sep;66(3):756-67.
Figures