3241
Heart-Rate Independent T2 Mapping for Overcoming Loss of BOLD Sensitivity in Conventional Cardiac T2 MRI Acquired Under Vasodilator StressHsin-Jung yang1, Damini Dey1, Jane Sykes2, John Butler2, Xiaoming Bi3, Behzad Sharif1, Ivan Cokic1, Sotirios Tsaftaris4, Debiao Li1, Piotr Slomka1, Frank Prato2, and Rohan Dharmakumar1
1Cedars Sinai Medical Center, Los Angeles, CA, United States, 2Lawson Health Research Institute, 3Siemens Healthcare, 4IMT School for Advanced Studies Lucca
Synopsis
Despite the advances to date, myocardial BOLD MRI continues to be plagued by imaging confounders, which limit its reliability. We hypothesized that (a) the loss in BOLD sensitivity is dependent on the magnitude of the change in heart rate (HR) between rest and vasodilator stress; and (b) HR-insensitive T2 maps can enable BOLD changes to be accurately captured. We tested our hypothesis by examining the BOLD response to a HR-insensitive T2 mapping approach and conventional T2 mapping. Our results show that reliability of T2-based myocardial BOLD MRI could be markedly improved through heart-rate-insensitive T2 acquisitions.
Introduction
Myocardial blood-oxygen-level-dependent (BOLD) MRI is a non-contrast approach for examining myocardial perfusion. Despite the advances to date, myocardial BOLD MRI continues to be plagued by imaging confounders, which can limit its reliability. Unlike most applications that rely on cardiac T2 MRI, myocardial BOLD MRI is acquired at rest and under vasodilator stress, which is often accompanied with an increase in heart rate (HR). We hypothesized that (a) the loss in BOLD sensitivity is directly dependent on the magnitude of the change in HR (ΔHR) between rest and vasodilator stress; and (b) HR-insensitive T2 maps can enable BOLD changes to be accurately captured. We tested our hypothesis by examining the BOLD response to a HR-insensitive T2 mapping approach and conventional T2 mapping. To assess whether ΔHR leads to a loss in myocardial BOLD sensitivity, we performed numerical simulations, ex-vivo imaging subjected to simulated ΔHR, and in-vivo imaging during adenosine infusion in health dogs.Methods
A HR-insensitive (saturation-recovery (SR) prepared), free-breathing 3D T2 mapping sequence at 3T with near perfect imaging efficiency was developed and studied in a hybrid clinical PET/MR system. Computer simulations employing Bloch equations, with parameters corresponding to commercially available 2D T2 mapping sequence (bSSFP readouts with Cartesian trajectory, TR/TE = 2.9 ms/1.1 ms, iPAT=2, partial Fourier = 3/4, FA = 35°, BW = 1184 Hz/ pixel, trigger pulse = 5, FOV = 288x360 mm2, matrix size =154x192, and voxel size = 2.5 x 1.7 x 6.0 mm3) and the proposed 3D T2 mapping approach (GRE readout with stack-of-stars trajectory, TR/TE = 3.0 ms/1.5 ms, flip angle (FA) = 15°, BW = 1100 Hz/ pixel, trigger pulse = 1, and voxel size = 2.0 x 2.0 x 6.0 mm3, SR recovery time= 130ms.), with and without SR preparation were performed to assess the dependence of heart rate on T2 measurements. To validate the simulation results and to experimentally determine the influence of heart rate on T2 maps, freshly excised canine hearts (n=3) were immersed in saline solution and individually scanned in a head coil with i) a commercially available 2D T2 mapping sequence, ii) the proposed 3D method without SR preparation and iii) proposed 3D sequence (which includes SR preparation) by artificially imposing heart rates of 40 – 110 beats/min (bpm) in increments of 10 bpm. 3D sequences were prescribed with full LV coverage and 2D sequences were prescribed to the match the 3D partitions. Heart rates were chosen to capture the typically observed DHR between conditions of rest and adenosine stress. In-vivo imaging was performed in healthy dogs (n=10). Conventional 2D T2 mapping, and proposed sequence were prescribed to investigate the influence of measured T2 by the HR elevation caused by adenosine stress. Hyperemic response from the in-vivo studies were validated with simultaneously acquired 13N-NH3 PET perfusion. Myocardial BOLD Response (MBR) was computed as 100% x [T2(stress)–T2(rest)]/ T2(rest), where T2(rest) and T2(stress) are the mean myocardial T2 pre- and post adenosine infusion. BOLD response derived from the conventional T2 map and the proposed HR-independent approach were compared to ass the loss in BOLD response from HR elevation during stress scans.Results
Numerical simulations and ex-vivo studies demonstrated that the proposed approach minimized the HR-dependent changes in T2 between rest and stress compared to the T2 maps acquired using conventional and proposed method without SR preparation (Fig. 1). T2 values acquired using the proposed sequence under adenosine stress was significantly greater than at rest (38.5±1.0 ms (rest) vs. 44.4±3.1 ms (stress), p<0.05), which was consistent with the PET perfusion: (0.8±0.1 ml/mg/min (rest) vs 2.0±0.9 ml/mg/min (stress); p<0.05, Fig. 2A and 2B). The discrepancy between MBR acquired with the proposed method and conventional T2 mapping, referred to here as Loss of Apparent BOLD Contrast [100% x (MBRprop – MBRconv)], was highly correlated (R=0.7, p<0.05) as shown in Fig. 2C.Conclusion
Conventional cardiac T2-based MRI is highly sensitive to ΔHR between rest and adenosine stress. These changes appear to decrease the expected BOLD response by counteracting the increase in T2 from vasodilator-induced hyperemia. The reliability of T2-based cardiac BOLD MRI could be improved through heart-rate-insensitive T2 acquisitions.Acknowledgements
No acknowledgement found.References
No reference found.Figures
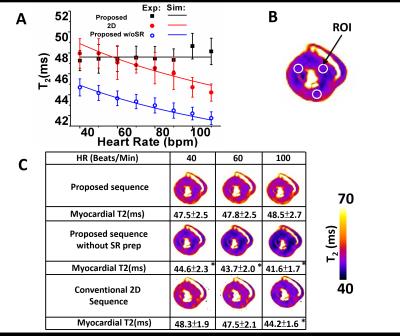
T2 dependence on heart rate as determined from
computer simulations and ex-vivo studies. In panel A, the theoretical(solid
lines) and experimental(dots) dependence of T2 on heart rate are shown for the
conventional 2D and proposed 3D T2 mapping with and without SR preparation. Panel
B shows representative ROIs used for the analysis. In panel C, representative
mid ventricular T2 maps acquired under different sequences and heart rates are
shown. Diminishing T2 values with increasing heart rates, as observed in
conventional 2D T2 maps and 3D T2 maps without SR preparation, were markedly
reduced with the proposed approach. * denotes p<0.05
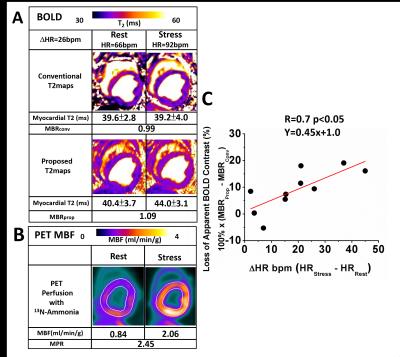
Dependence
of MBR Sensitivity on Heart Rate Differences Between Rest and Vasodilator
Stress. Representative rest and stress T2 maps obtained with conventional and
proposed sequences are shown in panel A. Myocardial T2 under stress with the
proposed T2 mapping approach is much higher than the T2 at rest, but T2 under
stress is reduced in the conventional images compared to rest. Corresponding
PET perfusion images are presented in panel B. Panel C shows the relationship
between Loss of Apparent MBR from conventional T2 map and HR increases. A strong correlation is observed
between Loss of Apparent MBR and ΔHR.