3240
Accelerated Cardiac Perfusion MRI with Radial k-space Sampling, Compressed Sensing, and KWIC filtering to Enable Qualitative and Quantitative Analyses of Perfusion.1Radiology, Northwestern University, Chicago, IL, United States, 2Biomedical Engineering, Northwestern University, Chicago, IL, United States, 3McCormick School of Engineering, Northwestern University, Chicago, IL, United States, 4Medicine, Northwestern University, Chicago, IL, United States
Synopsis
First-pass cardiac perfusion MRI is widely used as an important diagnostic tool for cardiovascular disease and extensive efforts are focused on improving spatial coverage, minimizing dark rim artifacts and quantifying absolute myocardial blood flow. In this study, we used a combination of radial k-space sampling, compressed sensing, and KWIC filtering to address these issues. Compared to the conventional perfusion technique, the accelerated method improved spatial coverage, minimized dark rim artifact and enabled quantification of myocardial blood flow.
Introduction:
First-pass cardiac perfusion MRI has been shown to be as accurate as SPECT in the diagnosis of coronary artery disease (CAD)1-4. Extensive on-going efforts in research are focused on improving spatial coverage5-9, minimizing dark rim artifacts (DRA)10-12, and enabling quantification of myocardial blood flow13, 14, all of which are not intersecting goals. A combination of radial k-space sampling and compressed sensing (CS)15 can be used to increase spatial coverage and minimize DRA9, 16. The k-space weighted image contrast (KWIC) filtering17, 18 can be used to retrospectively choose a unique saturation recovery time and perform simultaneous reconstruction with short recovery time for arterial input function (AIF) assessment and long recovery time for maximal tissue enhancement (e.g., dual imaging19-21). In this study, we sought to accomplish all three goals using a combination of radial k-space sampling, CS15, and KWIC filtering.Methods:
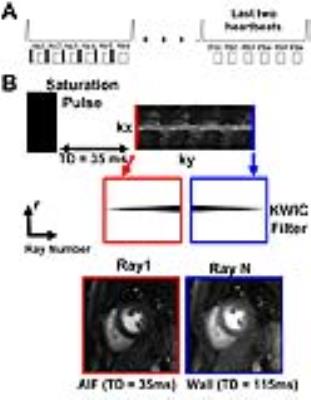
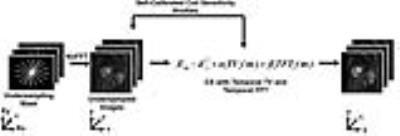
Pulse Sequence: We enrolled 7 patients (mean age = 55 ± 17; 3 males and 4 females) who were scheduled to undergo clinical cardiovascular MRI at 1.5T (Aera and Avanto, Siemens) and 3T (Skyra, Siemens), where standard cardiac perfusion MRI was performed as reference. Standard and 6.4-fold accelerated cardiac perfusion MRI scans were performed during free breathing with administration of 0.1 mmol/kg of Gadavist. To minimize the impact of residual contrast agent, we randomized the pulse sequence order. Imaging parameters for standard perfusion MRI included: TE/TR = 1.5/2.6 ms, slice thickness = 8 mm, acquisition matrix = 192x144, readout duration = 187.2 ms, saturation recovery time to the center of k-space (TI) = 136ms, 2-fold acceleration with TGRAPPA22, receiver bandwidth = 750 Hz/pixel, 3-4 short-axis planes per heartbeat, 65 repetitions, and flip angle = 15°. Similar parameters were used for the accelerated perfusion scans, except: acquisition matrix = 192 x 192, readout duration = 78 ms (30 rays), TI = 35ms for AIF and 115ms for wall enhancement, and 6-10 short-axis slices per heartbeat. For both the standard and accelerated perfusion scans, we additionally acquired proton density images without applying the saturation pulse at the back end of their respective first-pass perfusion scans, in order to convert signal intensity to contrast agent concentration, as previously described23 (Figure 1A). KWIC Filtering: To quantify AIF without signal saturation, images with a short saturation recovery time were reconstructed using a KWIC filter where the k-space center data was retained for the first ray and omitted for all other rays (Figure 1B, red). Signal modeling based on the Bloch equation describing saturation recovery was used to convert signal intensities to Gd concentrations23. For qualitative assessment, images with a longer saturation recovery time were reconstructed using a KWIC filter where the k-space center data was retained for the last ray (Figure 1B, blue). Undersampled images were reconstructed using CS with two orthogonal constraints in the same cost function: temporal total variance with normalized regularization weight = 0.05 and temporal fast Fourier transform with normalized regularization weight = 0.005 (Figure 2)24. Fourteen perfusion datasets (1 standard set and 1 CS set for 7 patients) were randomized and evaluated by 2 readers in a blinded, independent manner using a 5-point Likert scale for the following four categories: image quality, noise level, artifact level, and confidence level in ruling out DRA. Wilcoxon signed rank test was used to compare the scores (p <0.05 was considered statistically significant).Results
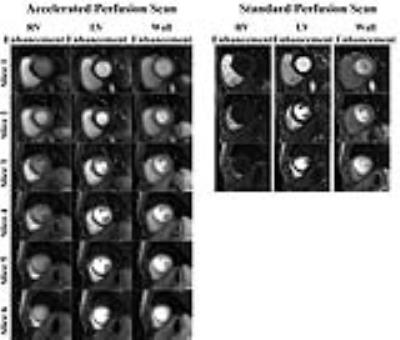
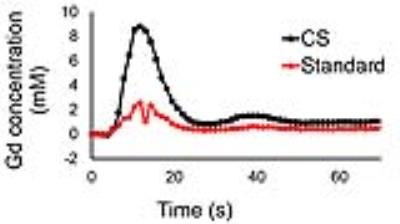
Figure 3 shows representative perfusion images acquired with the standard and accelerated pulse sequences. Note that CS enabled extensive spatial coverage with higher spatial and temporal resolution than conventional perfusion MRI. Table 1 summarizes the reader scores obtained for all 7 patients for the four categories. Confidence in ruling out DRA scores were significantly better for CS perfusion MRI as compared to standard perfusion MRI (p <0.05), whereas other scores were not significantly different between the two techniques. Figure 4 shows representative AIF (Gd-time) curves obtained from standard and accelerated perfusion data. AIF obtained using standard perfusion MRI sequence is underestimated as compared to AIF obtained using the accelerated perfusion MRI sequence. Averaging the results over 7 patients, mean peak Gd concentration from AIF curves was found to be 8.2 ± 2.3 mM using the accelerated technique with KWIC filtering.Conclusion
This study describes a novel approach that combines radial k-space sampling, CS, and KWIC filtering to improve the spatial coverage, minimize DRA, and enable quantification of myocardial blood flow. A limitation of this study is that only 7 patients were examined. A future study is warranted to evaluate the diagnostic performance in a diverse set of patients suspected with coronary artery disease.Acknowledgements
Grant Sponsor: 1R01HL116895-01A1References
1. Greenwood JP, Maredia N, Radjenovic A, Brown JM, Nixon J, Farrin AJ, Dickinson C, Younger JF, Ridgway JP, Sculpher M, Ball SG and Plein S. Clinical evaluation of magnetic resonance imaging in coronary heart disease: the CE-MARC study. Trials. 2009;10:62.
2. Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, Bijsterveld P, Ridgway JP, Radjenovic A, Dickinson CJ, Ball SG and Plein S. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453-60.
3. Greenwood JP, Motwani M, Maredia N, Brown JM, Everett CC, Nixon J, Bijsterveld P, Dickinson CJ, Ball SG and Plein S. Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease (CE-MARC) Trial. Circulation. 2014;129:1129-38.
4. Ahmad IG, Abdulla RK, Klem I, Margulis R, Ivanov A, Mohamed A, Judd RM, Borges-Neto S, Kim RJ and Heitner JF. Comparison of stress cardiovascular magnetic resonance imaging (CMR) with stress nuclear perfusion for the diagnosis of coronary artery disease. J Nucl Cardiol. 2016;23:287-97. 5. Manka R, Vitanis V, Boesiger P, Flammer AJ, Plein S and Kozerke S. Clinical feasibility of accelerated, high spatial resolution myocardial perfusion imaging. JACC Cardiovasc Imaging. 2010;3:710-7.
6. Motwani M, Maredia N, Fairbairn TA, Kozerke S, Radjenovic A, Greenwood JP and Plein S. High-resolution versus standard-resolution cardiovascular MR myocardial perfusion imaging for the detection of coronary artery disease. Circulation Cardiovascular imaging. 2012;5:306-13.
7. Plein S, Schwitter J, Suerder D, Greenwood JP, Boesiger P and Kozerke S. k-Space and time sensitivity encoding-accelerated myocardial perfusion MR imaging at 3.0 T: comparison with 1.5 T. Radiology. 2008;249:493-500.
8. Otazo R, Kim D, Axel L and Sodickson D. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magnetic Resonance in Medicine. 2010;64:767-76.
9. Harrison A, Adluru G, Damal K, Shaaban AM, Wilson B, Kim D, McGann C, Marrouche NF and Dibella EV. Rapid ungated myocardial perfusion cardiovascular magnetic resonance: preliminary diagnostic accuracy. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2013;15:26.
10. Maredia N, Radjenovic A, Kozerke S, Larghat A, Greenwood JP and Plein S. Effect of improving spatial or temporal resolution on image quality and quantitative perfusion assessment with k-t SENSE acceleration in first-pass CMR myocardial perfusion imaging. Magnetic Resonance in Medicine. 2010;64:1616-1624.
11. Motwani M, Jogiya R, Kozerke S, Greenwood JP and Plein S. Advanced Cardiovascular Magnetic Resonance Myocardial Perfusion Imaging: High-Spatial Resolution Versus 3-Dimensional Whole-Heart Coverage. Circulation: Cardiovascular Imaging. 2013;6:339-348.
12. Sharif B, Arsanjani R, Shalev A, Dharmakumar R, Bairey Merz CN, Berman DS and Li D. Dark-rim-free ungated first-pass perfusion CMR with 3-Slice end-systolic imaging: initial experience. Journal of Cardiovascular Magnetic Resonance. 2014;16:1-2.
13. Mordini FE, Haddad T, Hsu LY, Kellman P, Lowrey TB, Aletras AH, Bandettini WP and Arai AE. Diagnostic Accuracy of Stress Perfusion CMR in Comparison With Quantitative Coronary Angiography: Fully Quantitative, Semiquantitative, and Qualitative Assessment. JACC Cardiovasc Imaging. 2014;7:14-22.
14. Patel AR, Antkowiak PF, Nandalur KR, West AM, Salerno M, Arora V, Christopher J, Epstein FH and Kramer CM. Assessment of advanced coronary artery disease: advantages of quantitative cardiac magnetic resonance perfusion analysis. J Am Coll Cardiol. 2010;56:561-9. 15. Lustig M, Donoho D and Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magnetic Resonance in Medicine. 2007;58:1182-95.
16. Sharif B, Dharmakumar R, LaBounty T, Shufelt C, Thomson LE, Merz NB, Berman DS and Li D. Eliminating dark-rim artifacts in first-pass myocardial perfusion imaging. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2013;15:O3.
17. Song HK and Dougherty L. k-space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magn Reson Med. 2000;44:825-32.
18. Winkelmann S, Schaeffter T, Koehler T, Eggers H and Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE transactions on medical imaging. 2007;26:68-76.
19. Kim D and Axel L. Multislice, dual-imaging sequence for increasing the dynamic range of the contrast-enhanced blood signal and CNR of myocardial enhancement at 3T. Journal of magnetic resonance imaging : JMRI. 2006;23:81-6.
20. Gatehouse PD, Elkington AG, Ablitt NA, Yang G-Z, Pennell DJ and Firmin DN. Accurate assessment of the arterial input function during high-dose myocardial perfusion cardiovascular magnetic resonance. Journal of Magnetic Resonance Imaging. 2004;20:39-45.
21. Hsu LY, Kellman P, Gatehouse P, Zuehlsdorff S, Glielmi CB, Groves DW, Aletras AH, Bandettini PW and Arai AE. Comparison of arterial input function measured from dual-bolus and dual-sequence dynamic contrast-enhanced cardiac magnetic resonance imaging. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2011;13:O8.
22. Breuer FA, Kellman P, Griswold MA and Jakob PM. Dynamic autocalibrated parallel imaging using temporal GRAPPA (TGRAPPA). Magn Reson Med. 2005;53:981-5.
23. Cernicanu A and Axel L. Theory-based signal calibration with single-point T1 measurements for first-pass quantitative perfusion MRI studies. Academic radiology. 2006;13:686-93.
24. Feng L, Srichai MB, Lim RP, Harrison A, King W, Adluru G, Dibella EV, Sodickson DK, Otazo R and Kim D. Highly accelerated real-time cardiac cine MRI using k-t SPARSE-SENSE. Magn Reson Med. 2013;70:64-74.
Figures