3239
Validation of the Intravoxel Incoherent Motion Model in the Healthy Human Heart using Black-Blood Preparation1Institute for Biomedical Engineering, ETH Zürich, Zürich, Switzerland
Synopsis
In vivo cardiac Intravoxel Incoherent Motion Imaging (IVIM) offers the potential to estimate myocardial perfusion without the need for contrast agents. The IVIM parameter estimates, however, suffer from low signal-to-noise ratio, patient motion and are depending on imaging settings as well as on diffusion gradient shapes. In the present work, further evidence is presented that estimation of perfusion using a second-order motion compensated diffusion weighted sequence in the in vivo human heart is possible.
Introduction
The concept of Intravoxel Incoherent Motion (IVIM) imaging (1) in the heart has gained significant interest in recent years (2,3) as it provides a contrast agent free method for estimating tissue perfusion. The IVIM model has been extended for partially coherent blood flow (4,5) and applied to a number of studies in various organs (6,7). The impact of vascular perfusion on the diffusion weighted signal depends on imaging parameters and sequence design (4,8) and is hence subject to discussion.
The objective of the present work was to implement cardiac IVIM using a second-order motion compensated diffusion weighted spin-echo approach in conjunction with a dual inversion pre-pulse for blood suppression (black-blood) to validate the IVIM model in the beating human heart.
Methods
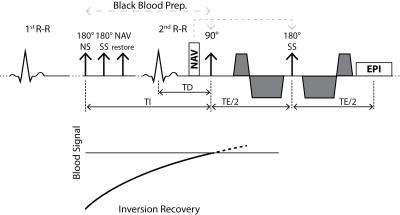
A second-order motion compensated diffusion weighted spin-echo EPI sequence (Figure 1) (9) was used on a 1.5T Philips Achieva system (Philips Healthcare, Best, The Netherlands) equipped with a 32-channel cardiac receiver coil array and a gradient system delivering 80mT/m at 100mT/m/ms.
Data from healthy volunteers (3 males, mean age 27years, mean weight 75kg, mean heart rate 61beats/min) were acquired. A short-axis slice at mid-ventricular level was prescribed and diffusion images were obtained with following parameters: spatial resolution: 2.4×2.4mm2, slice thickness: 10mm, reduced field-of-view (FOV): 230×105mm2, TR/TE: 2R-R/99ms, 4 signal averages, spectral-spatial water-only excitation. Diffusion encoding was performed using 15 b-values (range: 0-300s/mm2) acquired along six diffusion encoding directions (10) during cardiac contraction (50% end systole). Imaging was performed during free breathing with automatic slice tracking by using a navigator pencil beam on the right hemi-diaphragm. The total scan time was 11:20min at a heart rate of 60bpm.
In order to assess the impact of perfusing blood in tissue, a switchable black-blood dual-inversion pulse was incorporated into the sequence (Figure 1). The inversion delay time TI in Eq. [1] was set to minimize longitudinal magnetization of blood at the excitation pulse of the imaging block:
$$TI=-T1\cdot log\left[1/2\cdot \left(1+exp(-TR/T1)\right)\right], \qquad [1]$$
where T1 is the longitudinal relaxation constant of blood (1441ms, (11)) and TR is set to two R-R intervals. Diffusion imaging with identical imaging parameters was repeated subsequently without black-blood preparation as reference.
In post-processing, image registration of diffusion weighted images was performed (12, 13) to correct for residual respiratory motion induced geometrical inconsistency. In addition, image intensities were corrected to account for variations of the effective repetition time TR as a result of varying heart rate. Complex averaging of the respective signal averages and diffusion directions was performed (14). For parameter regression, a modified segmented least-squares approach was implemented (15) in Matlab (Natick, MA). The signal S(b) was fitted using the IVIM model in Eq. [2] with reference intensity S0, diffusion encoding strength b, diffusion coefficient D, perfusion fraction f and pseudo-diffusion coefficient D*:
$$S(b)=S_0 \left[f\cdot exp(-bD^{*})+(1-f)\cdot exp(-bD)\right] \qquad [2]$$
Mean values of the IVIM parameters and ranges are reported. Mann-Whitney-U-Tests are used to test significanct differences between the two measurements.
Results
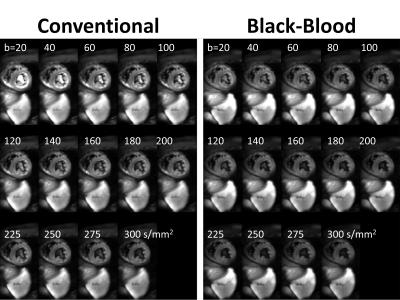
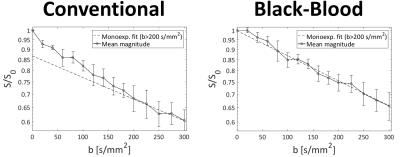
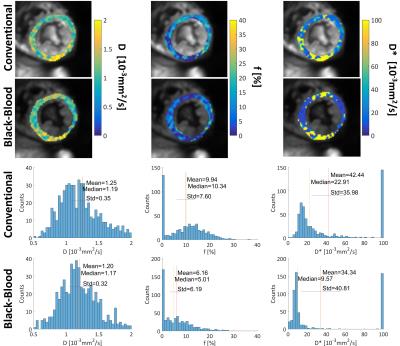
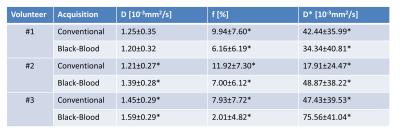
Systolic IVIM data were successfully acquired in all subjects (Figure 2). Example magnitude images and signal plots are shown in Figure 2 and 3. A biexponential signal decay can be observed for the conventional method, whereas the black-blood approach results in a monoexponential signal decrease. The diffusion coefficient remains within 15% relative difference among the conventional and the black-blood acquisition. The perfusion fraction however is reduced by 38-75% in the black-blood case, which is a significant reduction in all volunteers (P<0.05). The pseudo-diffusion coefficient D* exhibits a higher variance, which is due to the relatively large amount of fitting outliers. This can also be observed in the parameter maps and histograms in Figure 4.Discussion
Second-order motion compensated diffusion-weighted imaging allows to map an IVIM based perfusion estimate of the human heart during systolic contraction. The IVIM parameters in Figure 5 are in line with literature values (2). Imaging in systole reduces partial volume effects due to a more favorable ratio of voxel size to myocardial thickness when compared to imaging in diastole. In addition, systolic time points are more reproducible in the presence of heart rate variations. Black-blood preparation effectively suppresses the influence of blood flow through the capillaries on the magnitude signal.Conclusion
Intravoxel Incoherent Motion mapping is considered a promising approach to map myocardial perfusion estimates without the need for contrast agent administration. This study yields further evidence for the validity of the IVIM model in the human heart. Moreover, the proposed black-blood approach may be combined with diffusion (tensor) imaging methods to reduce confounding errors on diffusion metrics due to myocardial perfusion.Acknowledgements
This work is supported by VPH-DARE@IT and UK EPSRC (EP/I018700/1).References
1. Le Bihan D. Intravoxel incoherent motion imaging using steady-state free precession. Magn. Reson. Med. 1988;7:346–351.
2. Moulin KK, Croisille P, Feiweier T, Delattre BMA, Wei H, Robert B, Beuf O, Viallon M. In vivo free-breathing DTI and IVIM of the whole human heart using a real-time slice-followed SE-EPI navigator-based sequence: A reproducibility study in healthy volunteers. Magn. Reson. Med. 2016;76:70–82.
3. Delattre BMA, Viallon M, Wei H, Zhu YM, Feiweier T, Pai VM, Wen H, Croisille P. In vivo cardiac diffusion-weighted magnetic resonance imaging: quantification of normal perfusion and diffusion coefficients with intravoxel incoherent motion imaging. Invest. Radiol. 2012;47:662–670.
4. Wetscherek A, Stieltjes B, Laun FB. Flow-compensated intravoxel incoherent motion diffusion imaging. Magn. Reson. Med. 2015;74:410–419.
5. Karampinos DC, King KF, Sutton BP, Georgiadis JG. Intravoxel partially coherent motion technique: Characterization of the anisotropy of skeletal muscle microvasculature. J. Magn. Reson. Imaging 2010;31:942–953.
6. Marzi S, Piludu F, Forina C, Sanguineti G, Covello R, Spriano G, Vidiri A. Correlation Study between Intravoxel Incoherent Motion MRI and Dynamic contrast-enhanced MRI in Head and Neck Squamous Cell Carcinoma: Evaluation in Primary Tumors and Metastatic Nodes. Magn. Reson. Imaging 2016.
7. Federau C, Eth DP, Maeder P, Brien KO, Browaeys P. Quantitative Measurement of Brain Perfusion with Intravoxel. 2012;265:874-881.
8. Lin Y, Li J, Zhang Z, Xu Q, Zhou Z, Zhang Z, Zhang Y, Zhang Z. Comparison of Intravoxel Incoherent Motion Diffusion-Weighted MR Imaging and Arterial Spin Labeling MR Imaging in Gliomas. Biomed Res. Int. 2015;2015:1–10.
9. Stoeck CT, von Deuster C, Genet M, Atkinson D, Kozerke S. Second-order motion-compensated spin echo diffusion tensor imaging of the human heart. Magn. Reson. Med. 2016;75:1669–1676. doi: 10.1002/mrm.25784.
10. Jones DK, Horsfield M a., Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn. Reson. Med. 1999;42:515–525.
11. Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med 2005;54:507–512.
12. Vishnevskiy V, Gass T, Szekely G, Tanner C, Goksel O. Isotropic Total Variation Regularization of Displacements in Parametric Image Registration. IEEE Trans. Med. Imaging 2016;62:1–1.
13. Huizinga W. Groupwise Image Registration in Diffusion Weighted MRI using a PCA based Dissimilarity Metric Delft University of Technology Groupwise Image Registration in Diffusion Weighted MRI using a PCA based Dissimilarity Metric. 2013.
14. Scott AD, Nielles-Vallespin S, Ferreira PF, McGill L-A, Pennell DJ, Firmin DN. The effects of noise in cardiac diffusion tensor imaging and the benefits of averaging complex data. NMR Biomed. 2016;29:588–599.
15. Notohamiprodjo M, Chandarana H, Mikheev A, Rusinek H, Grinstead J, Feiweier T, Raya JG, Lee VS, Sigmund EE. Combined intravoxel incoherent motion and diffusion tensor imaging of renal diffusion and flow anisotropy. Magn. Reson. Med. 2015;73:1526–1532.
Figures