3237
Cardiac ASL using Single-Shot EPI at 3T1Electrical Engineering, University of Southern California, Los Angeles, CA, United States
Synopsis
Arterial spin labeling (ASL) is a non-contrast method for measuring tissue
Purpose
To determine the feasibility and potential advantages of single-shot EPI imaging in cardiac ASL.Introduction
Cardiac arterial spin labelling (ASL) is a non-contrast technique that is able to quantitatively assess myocardial blood flow (MBF) and detect angiographically significant coronary artery disease1. Current techniques provide only single-slice coverage, which is insufficient for clinical adoption, and has low temporal signal-to-noise ratio (tSNR) predominantly due to cardiac motion during imaging. Shorter imaging windows have been shown to improve tSNR2 and reduce sensitivity to heart rate (HR) variation. Single-shot EPI was among the earliest imaging schemes proposed for cardiac ASL at 1.5T3 and remains a promising alternative to balanced-SSFP imaging; it has the potential to reduce the imaging window from 200ms to 40ms without compromising spatial resolution. The short imaging window can potentially enable multi-slice imaging within stable diastole and improve tSNR (MBF/PN). This work takes inspiration from the recent and successful use of single-shot EPI for cardiac diffusion tensor imaging, another low SNR motion sensitive technique 4,5. In this work, we revisit and demonstrate the feasibility of myocardial ASL using single-shot EPI for systolic imaging at 3T.Methods
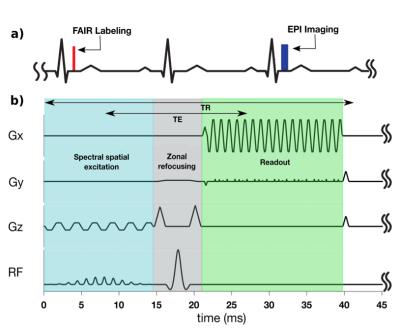
Experiments were performed on 9 healthy volunteers on a 3T GE (Signa Excite HD23) scanner with an 8-channel cardiac coil. Cardiac ASL measurements were performed using double-gated flow alternating inversion recovery (FAIR). The imaging sequence, shown in Figure 1, consists of a spectral-spatial excitation (to suppress fat), zonal refocusing6 (to reduce FOVy to 12.5 cm) , and single-shot partial-Fourier EPI (68% in ky). The crusher gradients around the spin echo pulse simultaneously suppress signal from flowing blood above a velocity cutoff, Vc. Conventional computation of Vc assumes a uniform velocity profile7. $$$ V_c=\frac{\pi}{2 \gamma A \delta}$$$,where $$$\gamma$$$ is the gyromagnetic ratio, A is the area of each crusher, and $$$\delta$$$ is the time difference between center of each crusher.
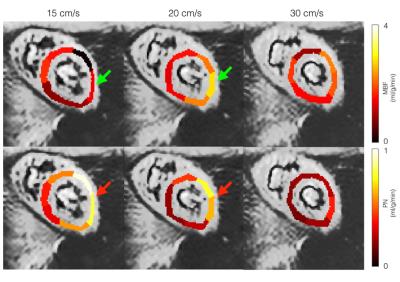
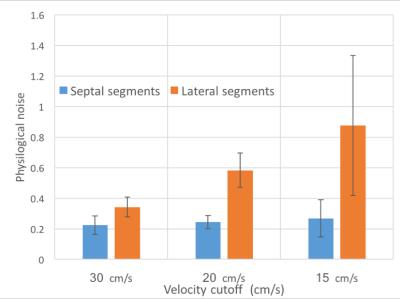
Imaging parameters were TR/TE=42ms/18ms, resolution=2.5x2.5mm, matrix size=192x50, phase-encodes=32, and readout time=20ms. EPI phase correction was performed using GE Orchestra and coil images were combined using optimal B1 coil combination8. Each scan consisted of 6 breath-holds of control and labeled image pairs. In 3 volunteers, the FAIR-EPI scans were repeated with multiple velocity cutoffs of 15, 20, and 30 cm/s to study its effect on estimated MBF, and PN. MBF and PN were measured in the left ventricular myocardium using double gated flow quantification3 and mapped onto the myocardium for global and regional analysis9.
Results and Discussion
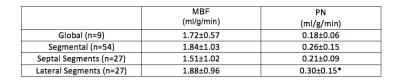
Table 1 summarizes the measured performance of FAIR-EPI. Global and segmental MBF were 1.72 ± 0.57 and. 1.84 ± 1.03 respectively using FAIR-EPI and 1.48 ± 0.45 and 1.54 ± 0.67, using FAIR-SSFP2. As shown in Table 1, PN was higher on average in the lateral wall (p< 0.05) and may account for the overestimation of MBF using FAIR-EPI. We hypothesize that this may be due to spurious labeling of the lateral wall, which has a higher degree of motion compared to the septum10. Spurious labeling can occur even at relatively high Vc because of the sinc shaped signal saturation vs. max velocity profile7. This imperfection in velocity profile can lead to partial saturation of moving myocardial spins even at lower velocities than Vc and confound ASL measurements which are only 1-4% of the image signal. This hypothesis is supported in Figure 2 and 3 and could be mitigated by applying the motion dephasing crushers on the y- or x-axis instead of the z-axis because the longitudinal myocardial velocity is higher than the radial or tangential velocity during systole10.Conclusion
The performance of FAIR-EPI on current hardware during end-systole was comparable to FAIR-SSFP during mid-diastole. We found that PN was higher in the lateral wall when compared to the septum and was dependent on Vc. Further work is needed to fully characterize the influence of Vc on the ASL signal, and to explore sequential multi-slice imaging.Acknowledgements
This work was funded in part by National Institutes of Health #R01-HL130494; American Heart Association #13GRNT13850012; Wallace H. Coulter FoundationReferences
[1] Zun Z, Wong EC, Nayak KS. Assessment of myocardial blood flow (MBF) in humans using arterial spin labeling (ASL): feasibility and noise analysis. Magn Reson Med. 2009 Oct;62(4):975-83.
[2] Do HP, Jao TR, Nayak KS. Myocardial arterial spin labeling perfusion imaging with improved sensitivity. J Cardiovasc Magn Reson. 2014 Jan 27;16:15.
[3] Poncelet BP, Koelling TM, Schmidt CJ, Kwong KK, Reese TG, Ledden P, Kantor HL, Brady TJ, Weisskoff RM. Measurement of human myocardial perfusion by double-gated flow alternating inversion recovery EPI. Magn Reson Med. 1999 Mar;41(3):510-9.
[4] Lau AZ, Tunnicliffe EM, Frost R, Koopmans PJ, Tyler DJ, Robson MD. Accelerated human cardiac diffusion tensor imaging using simultaneous multislice imaging. Magn Reson Med. 2015 Mar;73(3):995-1004.
[5] Ferreira PF, Kilner PJ, McGill LA, Nielles-Vallespin S, Scott AD, Ho SY, McCarthy KP, Haba MM, Ismail TF, Gatehouse PD, de Silva R, Lyon AR, Prasad SK, Firmin DN, Pennell DJ. In vivo cardiovascular magnetic resonance diffusion tensor imaging shows evidence of abnormal myocardial laminar orientations and mobility in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2014 Nov 12;16:87.
[6] Feinberg DA, Hoenninger JC, Crooks LE, Kaufman L, Watts JC, Arakawa M. Inner volume MR imaging: technical concepts and their application. Radiology. 1985 Sep;156(3):743-7.
[7] Wong EC, Cronin M, Wu WC, Inglis B, Frank LR, Liu TT. Velocity-selective arterial spin labeling. Magn Reson Med. 2006 Jun;55(6):1334-41.
[8] Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn Reson Med. 1990 Nov;16(2):192-225.
[9] T Jao, Z Zun, P Varadarajan, RG Pai, KS Nayak. "Myocardial ASL Data Filtering for Improved Detection of CAD," Proc. ISMRM 20th Scientific Sessions, Melbourne, May 2012, 3892.
[10] Jung B, Markl M, Föll D, Hennig J. Investigating myocardial motion by MRI using tissue phase mapping. Eur J Cardiothorac Surg. 2006 Apr;29 Suppl 1:S150-7.
Figures